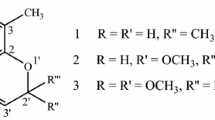Abstract
Two phenylpropanoid-conjugated iridoids, deglucosyl gaertneroside (1) and morindoidin (2), were isolated from the leaves of Morinda morindoides (Rubiaceae) by activity-guided fractionation using an anti-malarial activity assay. The known related iridoids molucidin (3) and prismatomerin (4), two lignans, abscisic acid, two megastigmanes, and two flavonol glycosides were also identified. The structures of isolated compounds were elucidated using spectroscopic analysis. The isolated compounds were evaluated for anti-malarial activity against the chloroquine/mefloquine-sensitive strains of Plasmodium falciparum together with cytotoxicity against adult mouse brain cells. Potent anti-malarial activity of 3 and 4 (IC50 of 0.96 and 0.80 μM, CC50 of 1.02 and 0.88 μM, and SI of 1.06 and 1.10, respectively) was shown, while new iridoids 1 and 2 and pinoresinol (5) displayed moderate activity (IC50 of 40.9, 20.6, and 24.2 μM) without cytotoxicity (CC50 > 50 μM). These results indicate that 1–5 may be promising lead compounds for anti-malarial drugs. In addition, our results imply the necessity of the quality control of the extract of M. morindoides leaves based on the contents of 1–5 in terms of the safety and efficacy.



Similar content being viewed by others
References
WHO (2020) World malaria report 2020. 20 years of global progress and challenges. Global malaria programme. WHO, Geneva, pp 1–65
Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415:680–685. https://doi.org/10.1038/415680a
Winstanley PA (2000) Chemotherapy for falciparum malaria: the armoury, the problems and the prospects. Parasitol Today 16:146–153. https://doi.org/10.1016/s0169-4758(99)01622-1
Murakami N, Umezome T, Mahmud T, Sugimoto M, Kobayashi M, Wataya Y, Kim H-S (1998) Anti-malarial activities of acylated bruceolide derivatives. Bioorg Med Chem Lett 8:459–462. https://doi.org/10.1016/S0960-894X(98)00045-6
Kim H-S, Shibata Y, Ko N, Ikemoto N, Ishizuka Y, Murakami N, Sugimoto M, Kobayashi M, Wataya Y (2000) Potent in vivo antimalarial activity of 3,15-di-O-acetylbruceolide against Plasmodium berghei infection in mice. Parasitol Int 48:271–274. https://doi.org/10.1016/s1383-5769(99)00023-9
Murakami N, Mostaqul HM, Tamura S, Itagaki S, Horii T, Kobayashi M (2001) New anti-malarial flavonol glycoside from hydrangeae dulcis folium. Bioorg Med Chem Lett 11:2445–2447. https://doi.org/10.1016/S0960-894X(01)00467-X
Murakami N, Sugimoto M, Kawanishi M, Tamura S, Kim H-S, Begum K, Wataya Y, Kobayashi M (2003) New semisynthetic quassinoids with in vivo antimalarial activity. J Med Chem 46:638–641. https://doi.org/10.1021/jm0201971
Kerharo J, Adam JG (1974) La pharmacopée sénégalaise traditionnelle. Plantes médicinales et toxiques. J Agric Trop Bot Appl 21:76–77
Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, Apers S, Hernans N, Van Miert S, Pieters L, Totté J, Vlietinck AJ (2004) In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J Ethnopharmacol 93:27–32. https://doi.org/10.1016/j.jep.2004.02.022
Abdoulaye T, Souleymane M, Howele O, Rene SY, Joseph DA, Adama C (2017) Comparative antimicrobial effectiveness of two medicated soaps with herbal soap from Morinda morindoides (Rubiaceae) against skin pathogens. Am J Microbiol Res 5:74–77. https://doi.org/10.12691/ajmr-5-4-1
Touré A, Bahi C, Ouattara K, Djama JA, Coulibaly A (2011) Phytochemical screening and in vitro antifungal activities of extracts of leaves of Morinda morindoides (Morinda, Rubiaceae). J Med Plant Res 5:6780–6786. https://doi.org/10.5897/JMPR11.1116
Cimanga K, De Bruyne T, Lasure A, Li Q, Pieters L, Claeys M, Vanden Berghe D, Kambu K, Tona L, Vlietinck A (1995) Flavonoid O-glycosides from the leaves of Morinda morindoides. Phytochemistry 38:1301–1303. https://doi.org/10.1016/0031-9422(94)00784-Q
Cimanga K, Hermans N, Apers S, Van Miert S, Van Den Heuvel H, Claeys M, Pieters L, Vlietinck A (2003) Complement-inhibiting iridoids from Morinda morindoides. J Nat Prod 66:97–102. https://doi.org/10.1021/np020215h
Seri CS, Ramiarantsoa H, Okpekon TA, Nana F, Dick A, Djakouré LA (2017) Two new iridoid glycosides from Morinda morindoides (Rubiaceae). J Pharm Phytochem 6:01–04
Cimanga RK, Tona GL, Kambu OK, Mesia GK, Muyembe JJT, Apers S, Pieters L, Vlietinck AJ (2008) Antimalarial activity of some extracts and isolated constituents from Morinda morindoides leaves. J Nat Rem 8:191–202
Seri CS, Ramiarantsoa H, Okpekon TA, Kabran FA, Kassi BBA, Fanté B, Djakouré LA, Coustard J-M (2018) A new ketosteroid from Morinda morindoides (Rubiaceae). J Pharm Phytochem 7:2403–2407
Cimanga K, De Bruyne T, Lasure A, Van Poel B, Pieters L, Vanden Berghe D, Vlietinck A, Kambu K, Tona L (1995) In vitro anticomplementary activity of constituents from Morinda morindoides. J Nat Prod 58:372–378. https://doi.org/10.1021/np50117a005
Cimanga K, De Bruyne T, Hu JP, Cos P, Apers S, Pieters L, Tona L, Kambu K, Vanden Berghe D, Vlietinck AJ (1999) Constituents from Morinda morindoides leaves as inhibitors of xanthine oxidase and scavengers of superoxide anions. Pharm Pharmacol Commun 5:419–424. https://doi.org/10.1211/146080899128735009
Cimanga RK, Kambu K, Tona L, Hermans N, Apers S, Totté J, Pieters L, Vlietinck AJ (2006) Cytotoxicity and in vitro susceptibility of Entamoeba histolytica to Morinda morindoides leaf extracts and its isolated constituents. J Ethnopharmacol 107:83–90. https://doi.org/10.1016/j.jep.2006.02.010
Cimanga RK, Mukenyi PN, Kambu OK, Tona GL, Apers S, Totté J, Pieters L, Vlietinck AJ (2010) The spasmolytic activity of extracts and some isolated compounds from the leaves of Morinda morindoides (Baker) Milne-Redh. (Rubiaceae). J Ethnopharmacol 127:215–220. https://doi.org/10.1016/j.jep.2009.11.018
Tamura S, Kubata BK, Syamsurizal IS, Horii T, Taba MK, Murakami N (2010) New anti-malarial phenylpropanoid conjugated iridoids from Morinda morindoides. Bioorg Med Chem Lett 20:1520–1523. https://doi.org/10.1016/j.bmcl.2010.01.095
Cimanga K, Kambu K, Tona L, Hermans N, Apers S, Totté J, Pieters L, Vlietinck AJ (2006) Antiamoebic activity of iridoids from Morinda morindoides leaves. Planta Med 72:751–753. https://doi.org/10.1055/s-2006-931581
Toume K, Hou ZY, Yu HH, Kato M, Maesaka M, Bai YJ, Hanazawa S, Ge YW, Andoh T, Komatsu K (2019) Search of anti-allodynic compounds from Plantaginis semen, a crude drug ingredient of Kampo formula “Goshajinkigan.” J Nat Med 73:761–768. https://doi.org/10.1007/s11418-019-01327-2
Teklemichael AA, Mizukami S, Toume K, Mosaddeque F, Kamel MG, Kaneko O, Komatsu K, Karbwang J, Huy NT, Hirayama K (2020) Anti-malarial activity of traditional Kampo medicine Coptis rhizome extract and its major active compounds. Malar J 19:1–10. https://doi.org/10.1186/s12936-020-03273-x
Yu HH, Toume K, Kurokawa Y, Andoh T, Komatsu K (2021) Iridoids isolated from Viticis Fructus inhibit paclitaxel-induced mechanical allodynia in mice. J Nat Med 75:48–55. https://doi.org/10.1007/s11418-020-01441-6
Tona L, Mesia K, Ngimbi NP, Chrimwami B, Okond’ahoka CK, De Bruyne T, Apers S, Hermans N, Totte J, Pieters L, Vlietinck AJ (2001) In vivo antimalarial activity of Cassia occidentalis Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol 95:47–57. https://doi.org/10.1080/00034983.2001.11813614
Trager W, Jenson JB (1978) Cultivation of malarial parasites. Nature 273:621–622. https://doi.org/10.1038/273621a0
Borenfreund E, Babich H (1987) In vitro cytotoxicity of heavy metals, acrylamide, and organotin salts to neural cells and fibroblasts. Cell Biol Toxicol 3:63–73. https://doi.org/10.1007/BF00117826
Mosaddeque F, Mizukami S, Kamel MG, Teklemichael AA, Dat TV, Mizuta S, Toan DV, Ahmed AM, Vuong NL, Elhady MT, Giang HTN, Dang TN, Fukuda M, Huynh LK, Tanaka Y, Egan TJ, Kaneko O, Huy NT, Hirayama K (2018) Prediction model for antimalarial activities of hemozoin inhibitors by using physicochemical properties. Antimicrob Agents Chemother 62:e02424-e2517. https://doi.org/10.1128/AAC.02424-17
Zirihi GN, Mambu L, Guédé-Guina F, Bodo B, Grellierd P (2005) In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol 98:281–285. https://doi.org/10.1016/j.jep.2005.01.004
Zirihi GN, N’guessan K, Dibié TE, Grellierd P (2010) Ethnopharmacological study of plant used to treat malaria, in traditional medicine, by Bete populations of ISSIA (Côte d’Ivoire). J Pharm Sci Res 2:216–227
Suzuki M, Tung NH, Kwofie KD, Adegle R, Amoa-Bosompem M, Sakyiamah M, Ayertey F, Owusu KB-A, Tuffour I, Atchoglo P, Frempong KK, Anyan WK, Uto T, Morinaga O, Yamashita T, Aboagye F, Appiah AA, Appiah-Opong R, Nyarko AK, Yamaoka S, Yamaguchi Y, Edoh D, Koram K, Ohta N, Boakye DA, Ayi I, Shoyama Y (2015) New anti-trypanosomal active tetracyclic iridoid isolated from Morinda lucida Benth. Bioorg Med Chem Lett 25:3030–3033. https://doi.org/10.1016/j.bmcl.2015.05.003
Krohn K, Gehle D, Dey SK, Nahar N, Mosihuzzaman M, Sultana N, Sohrab MH, Stephens PJ, Pan J-J, Sasse F (2007) Prismatomerin, a new iridoid from Prismatomeris tetrandra. Structure elucidation, determination of absolute configuration, and cytotoxicity. J Nat Prod 70:1339–1343. https://doi.org/10.1021/np070202+
Okunishi T, Umezawa T, Shimada M (2001) Isolation and enzymatic formation of lignans of Daphne genkwa and Daphne odora. J Wood Sci 47:383–388. https://doi.org/10.1007/BF00766790
Gohari AR, Saeidnia S, Bayati-Moghadam M, Amin G (2011) Lignans and neolignans from Stelleropsis antoninae. Daru 19:74–79
Abe F, Yamauchi T (1988) 9α-hydroxypinoresinol, 9α-hydroxymedioresinol and related lignans from Allamanda neriifolia. Phytochemistry 27:575–577. https://doi.org/10.1016/0031-9422(88)83144-3
Kim S-G, Hong I-P, Woo S-O, Jang H-R, Pak S-C, Han S-M (2017) Isolation of abscisic acid from Korean acacia honey with anti-Helicobacter pylori activity. Pharmacogn Mag 13:170–173. https://doi.org/10.4103/0973-1296.210166
Yan JK, Shi XL, Donkor PO, Zhu HJ, Gao XM, Ding L, Qiu F (2017) Nine pairs of megastigmane enantiomers from the leaves of Eucommia ulmoides Oliver. J Nat Med 71:780–790. https://doi.org/10.1007/s11418-017-1102-9
Almeida MFO, Melo ACR, Pinheiro MLB, Souza JRASADL (2011) Constituintes químicos e atividade Leishmanicida de Gustavia elliptica (Lecythidaceae). Quim Nova 34:1182–1187. https://doi.org/10.1590/S0100-40422011000700015
Lavoie S, Côté I, Pichette A, Gauthier C, Ouellet M, Nagau-Lavoie F, Mshvildadze V, Legault J (2017) Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis. BMC Complement Altern Med 17:123. https://doi.org/10.1186/s12906-017-1618-2
Han J-T, Bang M-H, Chun O-K, Kim D-O, Lee C-Y, Baek N-I (2004) Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch Pharm Res 27:390–395. https://doi.org/10.1007/bf02980079
Habila JD, Shode FO, Ndukwe GI, Amupitan JO, Nok AJ (2011) Novel antimalarial agent (cinnamic 3β-hydroxyolean-12-en-28-carboxylic anhydride): synthesis, characterization and in vivo studies. Afr J Pharm Pharmacol 5:2667–2675. https://doi.org/10.5897/AJPP11.589
Cimanga RK, Tona GL, Mesia GK, Kambu OK, Bakana DP, Kalenda PDT, Penge AO, Muyembe J-JT, Totté J, Pieters L, Vlietinck AJ (2006) Bioassay-guided isolation of antimalarial triterpenoid acids from the leaves of Morinda lucida. Pharm Biol 44:677–681. https://doi.org/10.1080/13880200601009123
Takenaka Y, Okazaki N, Nishi T, Nagakura N, Tanahashi T (2020) Iridoid glucosides from Linociera sangda. Heterocycles 101:186–194. https://doi.org/10.3987/COM-19-S(F)10
Ohta T, Tilkanont T, Ayertey F, Nakagawa M, Tung NH, Bolah P, Jnr HB, Appiah AA, Ocloo A, Ohashi M, Tanoue K, Yamaguchi Y, Ohta N, Yamaoka S, Iwanaga S, Uto T, Shoyama Y (2019) Establishment of a quantitative and qualitative analysis and isolation method for tetracyclic iridoids from Morinda lucida Bentham leaves. J Pharm Biomed Anal 164:475–480. https://doi.org/10.1016/j.jpba.2018.10.044
Acknowledgements
The authors would like to thank Mr. Vangu Kilukidi Blaise Van (University Hospital of Kinshasa, Faculty of Medicine, University of Kinshasa) and Dr. José Nzunzu Lami (Faculty of Pharmaceutical Sciences, University of Kinshasa) for providing the plant materials. This work was supported by the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University (2016-Ippan-20, 2017-Ippan-16, 2018-Ippan-20, and 2019-Ippan-22), a Grant-in-Aid for the Cooperative Research Project from the Institute of Natural Medicine, University of Toyama in 2017, and Kobayashi Foundation. YH is a recipient of a scholarship from Kyoritsu International Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
KT has received a research grant from Kobayashi Foundation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashim, Y., Toume, K., Mizukami, S. et al. Phenylpropanoid conjugated iridoids with anti-malarial activity from the leaves of Morinda morindoides. J Nat Med 75, 915–925 (2021). https://doi.org/10.1007/s11418-021-01541-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-021-01541-x