Abstract
Purpose
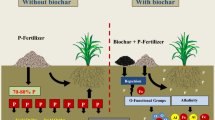
Biochar amendments can alter phosphorus (P) availability in soils, though the influencing mechanisms are not yet fully understood. This work investigated the adsorption and desorption of P on ferrihydrite (F, a Fe-oxide widely distributed in surface environments) in order to evaluate the interactions between P and Fe-oxide in the absence or presence of biochar (F or ferrihydrite–biochar (F–B) interaction) in soils.
Materials and methods
Biochar was produced by pyrolysis of rice straw at 600°C in steel ring furnaces. Two-line ferrihydrite was synthesized by dropwise addition of 1 mol L−1 KOH into Fe(NO3)3 solution until the pH reached 7–8 while stirring vigorously. An F–B complex was prepared under similar conditions, except that a mixture of 10 g biochar and the Fe(NO3)3 solution was used as the starting material instead of Fe(NO3)3 alone. A batch equilibration method was used to determine sorption or desorption of P. The mechanisms of P adsorption on F and F–B complex materials were discussed.
Results and discussion
Adsorption of P on F decreased as the pH was increased from 3.0 to 10, but the adsorption capacity of F decreased by about 30–40% in the presence of biochar. The P chemisorption rates on F also decreased in the presence of biochar. The Freundlich model showed that the active adsorption sites on the surface of the F–B complex were energetically heterogeneous. The desorbability of adsorbed P on F was enhanced by combination with biochar. The mechanisms of P adsorption on F and F–B complex materials are different.
Conclusions
The results showed that the amount and rate of P adsorption on the surface of ferrihydrite decreased with the presence of biochar, and the desorbability of adsorbed P on ferrihydrite can be enhanced when combined with biochar. Thus, the presence of biochar can decrease P adsorption on the Fe-oxides and enhance P availability in soils.






Similar content being viewed by others
References
Antelo J, Fiol S, Pérez C, Mariño S, Arce F, Gondar D, López R (2010) Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. J Colloid Interf Sci 347:112–119
Arai Y, Sparks DL (2001) ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. J Colloid Interf Sci 241:317–326
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Cao X, Ma L, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Chang MY, Juang RS (2005) Equilibrium and kinetic studies on the adsorption of surfactant, organic acids and dyes from water onto natural biopolymers. J Colloids Surf A: Physicochem Eng Aspects 269:35–46
Chen B, Yuan M (2011) Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J Soils Sediments 11:62–71
DeLuca TH, MacKenzie MD, Gundale MJ (2009) Biochar effects on soil nutrient transformation. Chapter 14. In: Lehmann J, Joseph S (eds) Biochar for environmental management science and technology. Earthscan, London, pp 251–280
Feng XH, Zhai LM, Tan WF, Liu F, He JZ (2007) Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ Pollut 147:366–373
Gupta SS, Bhattacharyya KG (2006) Adsorption of Ni(II) on clays. J Colloid Interf Sci 295:21–32
Guzman G, Alcantara E, Barron V, Torrent J (1994) Phytoavailability of phosphate adsorbed on ferrihydrite, hematite, and goethite. Plant Soil 159:219–225
Jaisi DP, Blake RE, Kukkadapu RK (2010) Fractionation of oxygen isotopes in phosphate during its interactions with iron oxides. Geochim Cosmochim Acta 74:1309–1319
Johnson SE, Loeppert RH (2006) Role of organic acids in phosphate mobilization from iron oxide. Soil Sci Soc Am J 70:222–234
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N (2010) An investigation into the reactions of biochar in soil. Aust J Soil Res 48:501–515
Khare N, Martin JD, Hesterberg D (2007) Phosphate bonding configuration on ferrihydrite based on molecular orbital calculations and XANES fingerprinting. Geochim Cosmochim Acta 71:4405–4415
Kolb SE, Fermanich KJ, Dornbush ME (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J 73:1173–1181
Kwon KD, Kubicki JD (2004) Molecular orbital theory study on surface complex structures of phosphates to iron hydroxides: calculation of vibrational frequencies and adsorption energies. Langmuir 20:9249–9254
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liu F, He J, Li X, Xu F, He H, Wang D (1995) Chemical states of phosphorus adsorbed on goethite at various phosphate concentrations. Chinese Sci Bull 40:506–511
Liu Y, Yang M, Wu Y, Wang H, Chen Y, Wu W (2011) Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J Soils Sediments. doi:10.1007/s11368-011-0376-x
Lou L, Wu B, Wang L, Luo L, Xu X, Hou J, Xun B, Hu B, Chen Y (2011) Sorption and ecotoxicity of pentachlorophenol polluted sediment amended with rice-straw derived biochar. Biores Technol 102:4036–4041
Luengo C, Brigante M, Avena M (2007) Adsorption kinetics of phosphate and arsenate on goethite. A comparative study. J Colloid Interf Sci 311:354–360
Namgay T, Singh B, Singh BP (2010) Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Aust J Soil Res 48:638–647
Novak JM, Busscher WJ, Laird DL, Ahmedna M, Watts DW, Niandou AS (2009) Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci 174:105–112
Parfitt RL, Atkinson RJ (1976) Phosphate adsorption on goethite (α-FeOOH). Nature 264:740–742
Rhoton FE, Bigham JM, Lindbo DL (2002) Properties of iron oxides in streams draining the Loess Uplands of Mississippi. Appl Geochem 17:409–419
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory: Preparation and characterization. Weinheim, Wiley-VCH Verlag GmbH
Wang H, Lin K, Hou Z, Richardson B, Gan J (2010) Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J Soils Sediments 10:283–289
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nature Commun 1:1–9
Yuan JH, Xu RK, Qian W, Wang RH (2011) Comparison of the ameliorating effects on an acidic ultisol between four crop straws and their biochars. J Soils Sediments. doi:10.1007/s11368-011-0365-0
Zeng L, Li X, Liu J (2004) Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res 38:1318–1326
Zheng W, Guo M, Chow T, Bennett DN, Rajagopalan N (2010) Sorption properties of greenwaste biochar for two triazine pesticides. J Hazard Mater 181:121–126
Zhong B, Stanforth R, Wu S, Chen JP (2007) Proton interaction in phosphate adsorption onto goethite. J Colloid Interf Sci 308:40–48
Acknowledgments
The authors gratefully acknowledge the two anonymous reviewers for their helpful comments and improving the English. This project was supported by the Science Foundation from the State Key Laboratory of Soil and Sustainable Agriculture (Y052010028), the National Natural Science Foundation of China (41001139), the Start-Up Foundation from the Institute of Urban Environment, Chinese Academy of Sciences (Y0L5611B60), and the Natural Science Foundation Project of CQ CSTC (CSTC, 2009BB1108).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Leo Condron
Rights and permissions
About this article
Cite this article
Cui, HJ., Wang, M.K., Fu, ML. et al. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J Soils Sediments 11, 1135–1141 (2011). https://doi.org/10.1007/s11368-011-0405-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0405-9