Abstract
Performance in endurance sports decreases with aging, which has been primarily attributed to cardiovascular and musculoskeletal aging; however, there is still no clear information on the factors that are most affected by aging. The aim of this study was to compare two groups of runners (< 50 and > 50 years of age) according to their absolute, weight-adjusted maximal oxygen uptake (V̇O2max), lower limb lean mass-adjusted V̇O2max, ventilatory threshold, and respiratory compensation point (RCP). A total of 78 male recreational long-distance runners were divided into Group 1 (38.12 ± 6.87 years) and Group 2 (57.55 ± 6.14 years). Participants were evaluated for body composition, V̇O2max, VT, and RCP. Group 1 showed higher absolute and body mass-adjusted V̇O2max (4.60 ± 0.57 l·min−1 and 61.95 ± 8.25 ml·kg−1·min−1, respectively) than Group 2 (3.77 ± 0.56 l·min−1 and 51.50 ± 10.22 ml·kg−1·min−1, respectively), indicating a significant difference (p < 0.001, d = − 1.46 and p < 0.001, d = − 1.16). Correspondingly, Group 1 showed a significantly higher lower limb lean mass–adjusted V̇O2max (251.72 ± 29.60 ml·kgLM−1·min−1) than Group 2 (226.36 ± 43.94 ml·kgLM−1·min−1) (p = 0.008, d = − 0.71). VT (%V̇O2max) (p = 0.19, d = 0.19) and RCP (%V̇O2max) (p = 0.24, d = 0.22) did not differ between the groups. These findings suggest that both variables that are limited by central or peripheral conditions are negatively affected by aging, but the magnitude of the effect is higher in variables limited by central conditions. These results contribute to our understanding of how aging affects master runners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is a well-known fact that performance in long-distance running events decreases with increasing age in a curvilinear manner [1]. Peak performance is generally achieved between the ages of 30 and 39 years, and only a modest decline occurs until the age of 50 years [2], coinciding with a significant muscle mass loss (~ 1.4% per year) after the age of 50 years [3].
One of the major determinants of long-distance exercise performance is the maximal oxygen uptake (V̇O2max), which is defined as the highest rate at which oxygen can be taken up by the lungs, transported by the cardiovascular system, and used by the muscular system [4]. Therefore, V̇O2max establishes the upper limit of maximal energy production through oxidative pathways [5]. In addition to V̇O2max, the percentage of V̇O2max that can be maintained during an endurance run, which is related to the V̇O2 measured at lactate threshold, is extremely important for long-distance exercise performance [4]. Finally, the energy demand to run at a given velocity, which has been termed running economy, is also an important determinant of performance, especially among athletes presenting similar V̇O2max values [6].
V̇O2max, which is an extremely important measurement reflecting the upper limit of endurance performance, is measured as an absolute rate of oxygen uptake per unit of time (l·min−1) in liters per minute, and there is a relative consensus in the literature that the major limiting factor of maximum V̇O2max is the central condition, i.e., the maximum cardiac output [7]. Although V̇O2max is measured in liters per minute, it can be expressed as a weight-adjusted rate (ml·kg−1·min−1), which is more adequate to compare individuals with different body masses [7]. This measure is also influenced by body composition, which becomes worse with increasing fat mass and decreasing lean mass during the aging process [8].
In this context, lower limb lean mass-adjusted V̇O2max, which has been termed “aerobic muscle quality,” is also essential for endurance performance and is gaining relevance [9]. As it measures the muscular capacity for oxygen consumption [10], lean mass-adjusted V̇O2max measurements are influenced by several peripheral conditions, such as mitochondrial enzyme levels and capillary density [9].
Although the decrease in aerobic performance with aging coincides with the decrease in V̇O2max values, the decline in V̇O2max may be greater than the decline in performance [1]. This situation emphasizes the effects of aging on all the physiological factors that affect aerobic performance, including the percentage of V̇O2max that can be sustained (i.e., aerobic endurance), which is generally measured using the anaerobic threshold (AT) and the respiratory compensation point (RCP), identified through a cardiopulmonary exercise test (CPET) [11].
Considering that the decline in performance due to aging is not of the same magnitude as the decline in V̇O2max absolute values, it is interesting to understand the magnitude of the impact of aging on other physiological variables that also affect performance but are limited by peripheral factors (e.g., lean mass-adjusted V̇O2max and AT) rather than central factors (e.g., absolute V̇O2max and body mass-adjusted V̇O2max).
Therefore, the present study was conducted to compare two groups of runners (< 50 and > 50 years of age) according to their absolute, weight-adjusted V̇O2max, lower limb lean mass-adjusted V̇O2max, and aerobic endurance variables (ventilatory threshold (VT) and RCP). We hypothesized that the physiological variables that are associated with performance and limited by central factors (absolute and weight-adjusted V̇O2max) would be more affected by the aging process than the variables that are limited by peripheral factors (aerobic endurance variables and lower limb lean mass-adjusted V̇O2max). Overall, this study emphasizes the importance of considering both central and peripheral factors in determining the effects of aging on running performance and provides a valuable resource for developing evidence-based training and testing interventions for runners of different ages and experience levels.
Materials and methods
Ethical approval
All experimental procedures were reviewed and approved by the Human Research Ethics Committee of the Federal University of Sao Paulo (approval number 4.354.386, October 22, 2020). The participants also signed a consent form after being provided with information regarding the study objectives, experimental procedures, risks, and benefits, as well as the assurance of privacy and confidentiality.
Participants
In total, 78 recreationally trained male runners were recruited through contact with running consultants, running coaches, training clubs, social media outreach, and partnership with sports medicine units. For analysis purposes, the participants were divided into two age groups, viz., Group 1 (20–49 years, n = 49) and Group 2 (50–70 years, n = 29). The age of 50 years was selected as the cutoff for both groups because after this age, the V̇O2max [1] and muscle strength [3] exhibit an accelerated decline. The characteristics of the participants are shown in Table 1.
The participants were required to have participated in a running training program for a minimum of 3 years, at least three times per week, and be aged between 20 and 70 years. To ensure study accuracy, only the participants with a BMI ≤ 29.9 kg/m2 were included because obesity can exert a negative impact on the variables under investigation, increase physical exertion, and potentially cause cardiovascular strain. Furthermore, obese individuals may have other health issues that could complicate study outcomes, further justifying their exclusion. In addition, participants should not have any medical contraindications to maximal effort exercise, smoking history, myopathies, systemic inflammatory disease, parenchymal lung disease, cardiomyopathy, peripheral vascular disease, or orthopedic conditions that would limit maximum performance in the assessments. Information regarding the training habits of participants is presented in Table 2. Only the participants who answered “NO” to all PAR-Q questions and did not have any contraindication to participation were eligible for the study. However, those who answered “YES” to at least one question, even if they were asymptomatic, were required to undergo a medical examination. The medical examination evaluated the family history of cardiovascular disease, relevant risk factors for coronary artery disease, risk classification, and signs of cardiovascular and pulmonary disease. Only with the approval of a physician were these individuals included in this study.
Study design
This was a cross-sectional study consisting of two visits to the exercise physiology laboratory during the morning period. During the first visit, an anamnesis was conducted using questionnaires, and during the second visit, body composition assessment and CPET were performed. The interval between visits was less than seven consecutive days to avoid any interference of the training routine in the research results. Participants were instructed to avoid engaging in intense exercise in the 24 h preceding their laboratory second visit and avoid eating 2 h before the test, but fasting was not required so that they could follow their regular hydration routine. They should wear suitable clothes and shoes for performing physical activities and avoid using any stimulating substance that could alter their performance in the tests, especially caffeine at least 8 h before the appointments.
Anamnesis
A custom questionnaire comprising 38 questions was developed to collect personal information, anthropometric data, and training characteristics. Participant’s body height and body mass were measured using a calibrated wall stadiometer and a balance (Filizola® São Paulo, Brazil) to the nearest 0.1 cm and 0.1 kg, respectively. The questionnaire consisted of the following three open questions: (1) How many years have you been running? (2) How many hours per week do you train? (3) Have you ever experienced any medical condition that could prevent you from engaging in physical activity in the last year? The participants also completed the Physical Activity Readiness Questionnaire (PAR-Q) [12].
Assessment of body composition
Body composition was evaluated using the dual-emission X-ray absorptiometry system (Software version 12.3, Lunar DPX, Wisconsin, USA). This technique involves minimal radiation exposure and is reliable for evaluating body composition [13]. Body mass, body fat, and lean body mass were measured for the whole body as well as for the lower and upper limbs and trunk. Lean mass and fat mass were expressed in absolute (kg) and relative (%) values.
Cardiopulmonary exercise testing
The CPET was conducted on a treadmill (Imbrasport, ATL, Brazil), and the collected data were used to determine VT, RCP, and metabolic responses. During the test, the participant’s physiological responses to exercise were measured using a metabolic system (Quark PFT; Cosmed®, Rome, Italy), which is a valid, accurate, and reliable tool for evaluating the abovementioned outcomes [14]. This system comprises a respiratory airflow sensor and rapid-response O2 and CO2 gas analyzers that were heat-adjusted and compensated for variations in barometric pressure, temperature, and relative humidity.
Breath samples were collected using a silicone face mask (V2Mask, Hans Rudolph Inc., USA), fixed with a cap supplied by the manufacturer, connected to a breathing valve, and transported to the analyzers through a semi-permeable Nafion® (synthetic polymer consisting of sulfhydryl residues) sample line that allowed rapid equilibrium with water vapor in the environment. Data processing was performed using the designated software (PFT, Cosmed®, Rome, Italy), compatible with the Windows® platform (USA). Calibration was performed before each test according to the manufacturer’s guidelines.
All evaluations started with 1 min of rest. If there was evidence of hyperventilation, which indicated anxiety and/or discomfort with the laboratory environment, the rest period was adjusted. This was followed by 3 min of warm-up at a constant speed and 3 min of exercise at 1.7 mph at a 10% grade. After the warm-up period, the test grade and speed were increased according to the Ellestad protocol [15]. The collection of gases for analysis was performed for each breath and then normalized every 20 s.
During the exercise period, all participants were encouraged to perform maximal progressive exercise until volitional exhaustion, as evaluated by symptoms such as dyspnea, severe fatigue, muscle pain, and inability to continue the test. The recovery period was 2 min, during which metabolic, cardiovascular, and respiratory variables were continuously monitored.
VT and RCP were determined using the ventilatory equivalents method [16]. VT was defined as the oxygen consumption corresponding to an effort intensity above in which a first increase in V̇E/V̇O2 is observed, i.e., a nonlinear increase in V̇E/V̇O2 with a concomitant increase in positive end-expiratory pressure of O2 (PEEPO2) and without an increase in V̇E/V̇CO2. RCP was defined as the oxygen consumption corresponding to an effort intensity above which an increase in V̇E/V̇CO2 with a concomitant decrease in positive end-expiratory pressure of CO2 (PEEPCO2) is observed.
V̇O2max was determined as the oxygen consumption achieved during maximal exercise intensity that could not be increased by < 150 ml·min−1 despite further increases in exercise intensity [17, 18]. If the participant did not meet this requirement, ventilatory, metabolic, and perceived exertion ratings were used to confirm that the test was maximal. These ratings consisted of the respiratory exchange ratio (RER) ≥ 1.10, (i.e., the ratio of the amount of CO2 being produced to the amount of O2 being consumed (V̇CO2/V̇O2)), if the participant reached the age-predicted maximum heart rate, and Borg’s perceived exertion scale = 20 [19]. Two experienced investigators independently identified VT and RCP. When there was no agreement on the independent identification, a third investigator settled the analysis. V̇O2max was measured as the absolute value (l·min−1), and then the total body mass-adjusted V̇O2max (ml·kg−1·min−1) and lower limb lean mass-adjusted V̇O2max (ml·kgLM−1·min−1) were calculated.
Statistical analysis
Descriptive data were expressed as mean, standard deviation, effect sizes, and effect size confidence intervals. Sample size was calculated considering a significant effect size of ≥ 0.51, a significance level of 0.05, and a power of 0.8. The calculation revealed that 98 subjects (49 per group) were required, but due to the COVID-19 outbreak and social distancing requirements, the planned data collection for the older age group could not be completed. An a posteriori analysis was performed based on the achieved effect size for the primary outcome (lower limb lean mass-adjusted V̇O2max expressed as ml·kgLM−1·min−1), which was 0.71, maintaining a significance level of 0.05 and a power of 0.8. The analysis revealed that at least 26 subjects per group would be sufficient. All data followed normal distribution according to the Shapiro–Wilk test, and homogeneity of variances was checked using Levene’s test. Differences between the two groups were investigated using Welch’s t-test because it performs better than Student’s t-test whenever sample sizes are different [20]. The measures of effect size for differences between groups were determined by calculating the mean difference between the two groups and then dividing the result by the pooled standard deviation. The magnitude of any change was judged based on the following criteria: d < 0.2 was considered to have no effect, 0.2 ≤ d < 0.5 was considered a small effect size, 0.5 ≤ d < 0.8 represented a medium effect size, 0.8 ≤ d < 1.3 indicated a large effect size, and d ≥ 1.3 was a very large effect size [21]. The level of significance was set at 0.05. All statistical analyses were performed using SPSS version 26.0 (SPSS, Inc., Chicago, IL, USA).
Results
Regarding running experience, as intentionally selected, older age runners (Group 2) had significantly more years of running experience (p < 0.001; d = 1.26 (large)). Moreover, the groups differed in terms of body composition, with significant differences found in the percentage of body fat (p = 0.001, d = 0.84 (large)), total lean mass, and lean mass of the lower limbs (p = 0.029, d = − 0.53 (medium), and p = 0.039, d = − 0.54 (medium), respectively). However, no differences were found in terms of total body mass (p = 0.902, d = 0.03), body height (p = 0.475, d = − 0.17), or BMI (p = 0.498, d = 0.16); the data are presented in Table 1.
To compare the training habits of participants, we compared their total mileage per week and number of running sessions per weekday and found no significant differences between the two groups (p = 0.292, d = − 0.23 (small), and p = 0.245, d = − 0.28 (small), respectively). However, the best 10 km time of the season reported by the participants showed a significant difference favoring the younger age group (p = 0.026, d = 0.66 (medium)); these data are shown in Table 2.
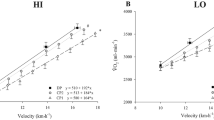
A comparison of the cardiorespiratory parameters obtained in the CPET between the groups is presented in Table 3.
Discussion
This study explored the differences in absolute, total body mass-adjusted, and lower limb lean mass-adjusted V̇O2max, VT, and RCP between two age groups of runners, i.e., < 50 and > 50 years. Our hypothesis was that the variables limited by central conditions, such as absolute and relative to body mass V̇O2max values, would be more affected by aging than the variables limited by peripheral conditions, such as relative to lean mass V̇O2max, VT, and RCP.
The major findings were as follows: (i) older age runners showed lower absolute and total body mass-adjusted V̇O2max (effect sizes were very large and large, respectively) than younger age runners; (ii) older age runners showed lower limb lean mass-adjusted V̇O2max (effect size was medium) than younger age runners; and (iii) VT (in %V̇O2max) and RCP (in %V̇O2max) showed no differences between older and younger age runners. Therefore, our initial hypothesis has been confirmed.
V̇O2max reflects an individual’s aerobic exercise capacity, which is a key indicator of physical fitness [22]. According to the Fick equation [23, 24], V̇O2max = maximal cardiac output (CO) × maximal oxygen arteriovenous difference (dif(a-v)O2), implying that a higher ability of the respiratory system to extract oxygen from the air, a higher ability of the cardiac system to transport oxygen to the exercising muscles, and a higher ability of the skeletal muscles to utilize oxygen, resulting in higher V̇O2max [7]. Although all these systems affect V̇O2max, the cardiovascular system, specifically cardiac systolic and diastolic function, is considered the central hub [25].
Our findings showed significantly lower V̇O2max (l·min−1) in runners aged > 50 years than in runners aged < 50 years, with a very large effect size. This decline in absolute V̇O2max in aging individuals is anticipated because there is an undeniable reduction in maximal CO with age [26, 27], causing a reduction in V̇O2max.
Aging also exerts a negative effect on muscle mass. A review by Mitchel et al. (2012) [28] showed that the median decline in muscle mass throughout an individual’s lifespan is 0.47% per year in men. Therefore, older age individuals have lower metabolically active tissue (skeletal muscle mass), which negatively impacts V̇O2max [29]. Although the older age runner group showed lower V̇O2max values than the younger age runner group, their V̇O2max (3.77 ± 0.56 l·min−1) was higher than the literature reference value for nonathletes aged 30 years (3.2 l·min−1) [30].
Despite the importance of absolute V̇O2max, this variable does not consider the body characteristics of an individual. Hence, the total body mass-adjusted V̇O2max shows a higher association with endurance performance [22], general health, and functional capacity [29]. In this context, elite male athletes typically have a V̇O2max between 70 and 85 ml·kg−1·min−1 [31], and subjects with < 14 ml·kg−1·min−1 are indicated for heart transplantation [32]. The cutoff for an independent lifestyle has been considered 18 and 15 ml·kg−1·min−1 in men and women, respectively [33].
The results of the present study also demonstrated a significant difference between the two age groups according to V̇O2max relative to body mass. Although the effect size was classified as large, it was lower than the effect size for absolute V̇O2max values. Despite the evident reduction in total body mass-adjusted V̇O2max, the older age runner group showed values much higher than expected for their age group, considering the literature data for healthy adults. According to a review based on 62 previous studies [30], V̇O2max values > 46 ml·kg−1·min−1 are considered excellent for men aged 50–55 years, and the older age group in the present study showed a mean V̇O2max value of 51.5 ml·kg−1·min−1. These data indicate that despite the decrease in V̇O2max with age, even in runners, the runners may live longer with higher V̇O2max values, which implies more years of independent life.
Another insightful method for normalization is expressing V̇O2max adjusted by the lean mass. Lean mass (skeletal muscle mass) is the largest component of the fat-free body mass in humans [34], which is required for athletic performance and also for an independent lifestyle for elderly individuals [28]. This variable should also be evaluated to examine physical fitness because reduced muscular aerobic capacity also contributes to reduced whole-body V̇O2max absolute values [35]. As lean mass-adjusted V̇O2max reflects the amount of oxygen consumed per kilogram of skeletal muscle, it has been termed “aerobic muscle quality” [9]. There are several suggested limiting factors for this variable, which are primarily peripheral conditions, such as intramuscular adipose tissue, capillary density, structural composition, and mitochondrial density [9, 36]. The older age group in this study showed significantly lower limb lean mass-adjusted V̇O2max than the younger age group; however, the effect size was medium, which was lower than the effect size for absolute and body mass-adjusted V̇O2max, evidencing a smaller impact of aging on the peripheral condition (muscular aerobic capacity) than on the central condition (CO).
Similarly, both VT and RCP (%V̇O2max) showed no significant differences between the two age groups. Similar to lower limb lean mass-adjusted V̇O2max, the percentage of V̇O2max that can be sustained is primarily associated with muscular conditions (such as the percentage of type I muscle fibers, mitochondrial level, motor unit recruitment, and firing rates) [7]. This supports the finding that the effects of aging are more pronounced on variables constrained by the central condition than on those constrained by the peripheral condition among runners.
Data from the literature show that the decline in V̇O2max due to aging is greater than the decrease in performance [1]. The results of the present study partially explain this phenomenon, because other performance-related variables, such as peripheral oxidative capacity, are less affected by aging than absolute V̇O2max.
Study limitations
There are some limitations in this study that must be considered. This study used a cross-sectional design, implying that the findings merely provide a momentary depiction of the participants’ physiological status. Further research implementing longitudinal studies is necessary to establish how these variables evolve over time. Moreover, the use of self-reported questionnaires for anamnesis is a potential source of recall bias.
Most importantly, another potential limitation of this study is the suggestion and use of comparisons of rates or proportions between the two groups to draw conclusions about differences in performance or outcomes. This approach can be influenced by rate difference bias, which occurs when a small difference in the underlying rates of the two groups is amplified using rates or proportions in the statistical analysis. This can cause an overestimation or underestimation of the true difference in performance or outcomes between the groups.
Practical applications
This study provides valuable insights into the differences in V̇O2max among young and old age runners with varying levels of experience. The results indicate that although absolute V̇O2max declines with age, V̇O2max relative to the lean mass of lower limbs is less affected in older age runners compared with absolute values. Our study has practical applications in designing training programs for runners of different ages and experience levels. It also adds to our knowledge of the effect of aging on the physiological variables that are strongly associated with health status. Coaches, trainers, and researchers could use these data to develop exercise programs that emphasize central or peripheral adaptations in runners, depending on the major physiological system affected by the aging process.
Conclusions
This study suggests that although the variables that affect endurance performance, which are limited by the central condition (absolute V̇O2max), are severely negatively affected by aging, other variables that are limited by the peripheral condition (lower limb lean mass-adjusted V̇O2max) are less affected by aging. Despite the limitation of the cross-sectional design used in this study, the findings could provide useful insights for understanding the effects of aging on the physiological determinants of aerobic performance and for the development of effective training programs and interventions for runners, particularly for master athletes who constitute an increasing category of practitioners in recent decades.
Data availability
We will make available the data upon request.
References
Tanaka H, Seals DR. Invited review: dynamic exercise performance in Masters athletes: insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol. 1985;2003(95):2152–62.
Mendonca GV, Pezarat-Correia P, Vaz JR, Silva L, Heffernan KS. Impact of aging on endurance and neuromuscular physical performance: the role of vascular senescence. Sports Med. 2016;47(4):583–98.
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–6. https://doi.org/10.1152/jappl20008841321.
Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84.
Saltin B, Astrand PO. Maximal oxygen uptake in athletes. J Appl Physiol. 1967;23:353–8.
Saunders PU, Pyne DB, Telford RD, Hawley JA. Factors affecting running economy in trained distance runners. Sports Med. 2004;34:465–85.
Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84.
Strasser B, Burtscher M. Survival of the fittest: VO 2 max, a key predictor of longevity? Front Biosci. 2018;23:1505–16.
Trinschek J, Zieliński J, Kusy K. Maximal oxygen uptake adjusted for skeletal muscle mass in competitive speed-power and endurance male athletes: changes in a one-year training cycle. Int J Environ Res Public Health. 2020;17:1–13.
Beekley MD, Abe T, Kondo M, Midorikawa T, Yamauchi T. Comparison of normalized maximum aerobic capacity and body composition of sumo wrestlers to athletes in combat and other sports. J Sports Sci Med. 2006;5 CSSI:13.
Bosquet L, Léger L, Legros P. Methods to determine aerobic endurance. Sports Med. 2002;32:675–700.
Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci. 1992;17:338–45.
Colyer SL, Roberts SP, Robinson JB, Thompson D, Stokes KA, Bilzon JLJJ, et al. Detecting meaningful body composition changes in athletes using dual-energy x-ray absorptiometry. Physiol Meas. 2016;37:596–609.
Nieman DC, Austin MD, Dew D, Utter AC. Validity of COSMED’s quark CPET mixing chamber system in evaluating energy metabolism during aerobic exercise in healthy male adults. Res Sports Med. 2013;21:136–45. https://doi.org/10.1080/154386272012757227.
Thomas GS, Wann LS, Ellestad MH. Ellestad’s stress testing. 6th ed. Oxford University Press; 2018.
Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35:236–43.
Wasserman K, Hansen J, Sue J, Stringer W, Whipp B, Sue D, et al. Principles of exercise testing and interpretation. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
ATS, ACCP. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77.
Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16:55–8.
Delacre M, Lakens D, Leys C. Why psychologists should by default use Welch’s t-test instead of Student’s t-test. Int Rev Soc Psychol. 2017;30:92–101.
Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4:279.
Puccinelli PJ, Lima GHO, Pesquero JB, de Lira CAB, Vancini RL, Nikolaids PT, Knechtle B, Andrade MS. Previous experience, aerobic capacity and body composition are the best predictors for Olympic distance triathlon performance: predictors in amateur triathlon. Physiol Behav. 2020;225:113110.
Whipp BJ, Ward SA. Quantifying intervention-related improvements in exercise tolerance. Eur Respir J. 2009;33:1254–60.
Weisman IM, Marciniuk D, Martinez FJ, Testing measurements Frank Sciurba E, Sue D, Myers Bruce Johnson J, et al. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77.
Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–19.
Luisada AA, Bhat PK, Knighten V. Changes of cardiac output caused by aging: an impedance cardiographic study. Angiology. 1980;31:75–81.
Rivera-Brown AM, Frontera WR. Principles of exercise physiology: responses to acute exercise and long-term adaptations to training. PM R. 2012;4:797–804.
Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260.
Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–211.
Shvartz E, Reibold R. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61:3–11.
Larsen HB, Sheel AW. The Kenyan runners. Scand J Med Sci Sports. 2015;25:110–8.
Mancini DM, Eisen H, Kussmaul W, Mull R, Edmonds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86.
Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med. 2009;43:342–6.
Kim J, Shen W, Gallagher D, Jones A, Wang ZM, Wang J, et al. Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. Am J Clin Nutr. 2006;84:1014–20.
Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J Appl Physiol. 1985;1997(82):1411–5.
Zhou N. Assessment of aerobic exercise capacity in obesity, which expression of oxygen uptake is the best? Sports Med Health Sci. 2021;3:138–47.
Acknowledgements
We greatly appreciate the volunteers who took the time to contribute to this study.
Funding
Open access funding provided by University of Zurich We also thank CAPES for providing financial support (Finance code 001), the Postgraduate Program in Translation Medicine, and the Olympic Center of Training and Research (COTP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Seffrin, A., Vivan, L., dos Anjos Souza, V.R. et al. “Impact of aging on maximal oxygen uptake adjusted for lower limb lean mass, total body mass, and absolute values in runners”. GeroScience 46, 913–921 (2024). https://doi.org/10.1007/s11357-023-00828-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00828-z