Abstract
Lysophosphatidylcholines (LPCs) are phospholipids critical in the synthesis of cardiolipin, an essential component of mitochondrial membranes. Lower plasma LPCs have been cross-sectionally associated with lower skeletal muscle mitochondrial function, but whether lower LPCs and their decline over time are longitudinally associated with an accelerated decline of mitochondria function is unknown. We analyzed data from 184 participants in the Baltimore Longitudinal Study of Aging (mean age: 74.5 years, 57% women, 25% black) who had repeated measures of plasma LPCs (16:0, 16:1, 17:0, 18:0, 18:1, 18:2, 20:3, 20:4, 24:0, and 28:1) by liquid chromatography-tandem mass spectrometry and repeated measures of skeletal muscle oxidative capacity (kPCr) assessed by 31P magnetic resonance spectroscopy over an average of 2.4 years. Rates of change in kPCr and each LPC were first estimated using simple linear regression. In multivariable linear regression models adjusted for baseline demographics and PCr % depletion, lower baseline LPC 16:1 and faster rates of decline in LPC 16:1 and 18:1 were significantly associated with a faster rate of decline in kPCr (B = − 0.169, 95% CI: − 0.328, − 0.010, p = 0.038; B = 0.209, 95% CI: 0.065, 0.352, p = 0.005; B = 0.156, 95% CI: 0.011, 0.301, p = 0.035, respectively). Rates of change in other LPCs were not significantly associated with change in kPCr (all p > 0.05). Lower baseline concentrations and faster decline in selected plasma lysophosphatidylcholines over time are associated with faster decline in skeletal muscle mitochondrial function. Strategies to prevent the decline of plasma LPCs at an early stage may slow down mitochondrial function decline and impairment during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondria are dynamic organelles producing most of the energy required for cellular metabolism and are often referred to as “the powerhouse of the cell.” Loss of mitochondrial function is crucial in the aging and disease processes. Lysophosphatidylcholines (LPCs), a major class of phospholipids, can have positive and negative effects on mitochondria under specific conditions. The effect of LPCs on mitochondria is complex and not fully understood (for review, see [8]. For instance, LPCs can promote the generation of reactive oxygen species (ROS) in mitochondria, which lead to increased oxidative stress and inflammation, especially in older age [7, 15, 17, 22, 30, 34] (for review, see [21]. LPCs also have anti-inflammatory properties [14, 15, 18, 20] (for review see [19]. For example, LPCs 16:0 and 18:1 can activate peroxisome proliferator–activated receptor δ which is involved in mitochondrial metabolism regulation and protects against fatty acid–induced inflammation in human skeletal muscle [18]. On the other hand, LPCs are important for the synthesis of cardiolipin, a diphosphatidylglycerol lipid unique to mitochondria and essential for mitochondrial function. Specifically, the breakdown products of LPCs, such as lysophosphatidic acid (LPA) and phosphatidic acid (PA), are key intermediates in the synthesis of cardiolipin [31]. Cardiolipin is a phospholipid specifically localized in the inner mitochondrial membrane, essential for mitochondrial bioenergetics and signaling pathways. Cardiolipin can shape the curvature of the mitochondrial cristae, affect the assembly and correct functioning of the electron transport chain complexes, and consequently alter ATP and ROS production by mitochondria [9, 28].
Recent data suggest that LPCs measured in the blood may be considered a biomarker of mitochondrial function in skeletal muscle. Lower concentrations of selected long-chain LPCs (i.e., chains composed of at least 12 carbons) are cross-sectionally associated with lower skeletal muscle mitochondrial function [32]. Whether a change in plasma levels of LPCs over time is associated with a parallel change in skeletal muscle mitochondrial function has not been investigated. Finding a longitudinal association may further support the hypothesis that LPCs blood levels are causally related to loss of mitochondrial function with aging, and suggest that LPCs may be targeted for intervention aimed at improving mitochondrial function and, in turn, related aging phenotypes. This hypothesis is consistent with previous studies suggesting that lower plasma concentration of selected LPCs, such as 17:0, 18:1, and 18:2, are also associated with aging phenotypes including slower gait speed and greater mobility decline in middle-aged and older adults [11, 27]. In this study, we aimed to examine longitudinal associations between blood LPCs and skeletal muscle mitochondrial function using data from the Baltimore Longitudinal Study of Aging. We hypothesized that baseline concentrations and changes of LPCs over time would be associated with changes in skeletal muscle mitochondrial function assessed in vivo by 31P-magnetic resonance spectroscopy (31P-MRS).
Methods
Study population
Participants were drawn from the Baltimore Longitudinal Study of Aging (BLSA), a longitudinal study with a continuous enrollment since 1958 [10, 33]. In brief, participants must be free of major chronic conditions and cognitive impairment at the time of enrollment. Follow-up visits occur at different intervals depending on a participant’s age (every 4 years for those younger than 60 years, every 2 years for those aged 60–79, and every year for those aged 80 or older). The National Institutes of Health Institutional Review Board approved the BLSA protocol. All participants provided written informed consent at each BLSA visit.
In this study, data were collected between August 2013 and September 2019. The first concurrent assessment of plasma LPCs and mitochondrial function was considered “baseline” in this analysis. We identified and analyzed 184 adult participants who had two or more measures of plasma LPCs and skeletal muscle mitochondrial function over a mean of 2.4 (SD = 0.9) years. The average number of visits was 2.3 (SD = 0.6) per participant.
Skeletal muscle oxidative capacity determined by 31P-MRS
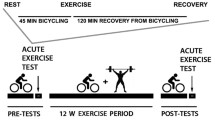
In vivo 31P-MRS measurements of phosphorus-containing metabolites were obtained from the quadriceps muscles using a 3 T Achieva MR scanner (Philips, Best, The Netherlands), as described in detail previously [4, 41].
Participants were positioned supine in the bore of the scanner with a foam wedge underneath the knee to maintain slight flexion (30°), with thighs and hips secured with straps to reduce displacement during exercise. As instructed, participants performed a fast, intense, ballistic knee extension exercise designed to deplete PCr in the quadriceps muscles with minimal acidification, permitting assessment of maximal oxidative phosphorylation [5].
A series of pulse-acquire 31P-MRS spectra were obtained before, during, and after exercise using a 10-cm 31P-tuned, flat surface coil (PulseTeq, Surrey, UK) that was secured over the vastus lateralis muscle of the left thigh. 31P nuclei were excited with 90° adiabatic radio frequency (RF) pulses with an inter-pulse delay time TR = 1.5 s, a four-step phase cycle, and four averages, resulting in a temporal resolution of 6 s. A total of 75 spectra were obtained over the 60 s before, 30 s during, and 360 s after exercise; the total duration of MR data acquisition was 7.5 min [4].
Spectra were processed using jMRUI (version 5.2) and fit in the time domain using a nonlinear least-squares algorithm (AMARES) [26, 37]. Maximum muscular oxidative capacity was assessed as the post-exercise PCr recovery rate constant, kPCr, which was determined by fitting time-dependent changes in PCr peak area using the following mono-exponential function:
where PCr0 was the PCr signal amplitude at the end of the exercise (i.e., the beginning of the recovery), ΔPCr was the decrease in PCr observed from baseline to the end of the exercise, τ was the PCr recovery time constant, and kPCr was the PCr recovery rate constant determined as 1/τ [4, 41]. To standardize the measure of oxidative function across different participants, the duration of exercise was carefully optimized by consistently requiring a reduction in PCr peak height of at least 33% compared with initial baseline values [25].
Collection of plasma
Blood was collected from participants at the National Institute on Aging Clinical Research Unit, Medstar Harbor Hospital in Baltimore, MD, following a standardized protocol, as described previously [40]. Participants were not allowed to smoke, exercise, or take medications before the blood samples were collected. Blood samples were drawn from the antecubital vein between 07:00 and 08:00 am after an overnight fast, then stored immediately at 4 °C, centrifuged within 4 h, and immediately aliquoted and frozen at − 80 °C. The collection of EDTA plasma in the BLSA is consistent with guidelines for biomarker studies [36].
Measurement of plasma metabolites
Plasma metabolites were measured using liquid chromatography with tandem mass spectrometry (LC–MS/MS). Metabolites were extracted and concentrations were measured using the MxP Quant 500 kit (Biocrates Life Sciences AG, Innsbruck, Austria) following the manufacturer’s protocol for a 5500 QTrap (Sciex, Framingham, MA, USA), as described in detail previously [40]. Of 14 LPCs potentially assessed by the MxP Quant 500 kit, 10 LPCs had all concentration values above the limit of detection and were included in this analysis.
Statistical analysis
Bivariate correlations of participants’ characteristics with kPCr at baseline and the last visit were examined using Pearson correlation coefficients for continuous variables and t-tests for categorical variables.
We first examined cross-sectional associations at baseline to confirm previous findings using the MxP Quant 180 kit [32]. We then estimated rates of change in kPCr and each LPC per participant using simple linear regression. To examine associations of baseline LPCs and rates of change in LPCs with the rate of change in kPCr, we used multivariable linear regression. Models were adjusted for baseline age, sex, race, height, and extent of baseline PCr depletion. In sensitivity analyses, we repeated the analysis by using relative measures of LPCs which were significant in the main analyses of the absolute concentration values. The relative measure was calculated as a ratio of the individual concentration to the sum of the concentrations from all 10 LPCs.
All analyses were performed using RStudio version 4.0.2 (Boston, MA). Significance was set at two-tailed p < 0.05.
Results
We analyzed 184 participants who had repeated measures of both plasma LPCs and skeletal muscle mitochondrial function, assessed as kPCr via 31P-MRS, over an average of 2.4 (SD = 0.9) years. The mean baseline age of participants in this sample was 74.5 years old, 57% were women, and 25% were black (Table 1). On average, the skeletal muscle mitochondrial function measure, kPCr, declined 0.00002 (SD = 0.002) per year in this analytical sample.
We first confirmed the cross-sectional associations published previously except for LPC 18:0 (Semba, et al. 2018). In our study, lower concentrations of several plasma LPCs (16:0, 16:1, 17:0, 18:0, 18:1, 18:2, and 20:3) were cross-sectionally associated with lower kPCr at baseline with and without covariate adjustment (Supplementary Table 1). Associations of kPCr with LPC 20:4, 24:0, and 28:1 were not significant at baseline (Supplementary Table 1).
In multivariable linear regression models, lower baseline concentration of baseline LPC 16:1 and faster rates of decline in LPC 16:1 and 18:1 were significantly associated with a faster rate of decline in kPCr with and without covariate adjustment (in adjusted models: B = − 0.169, 95% CI: − 0.328, − 0.010, p = 0.038; B = 0.209, 95% CI: 0.065, 0.352, p = 0.005; B = 0.156, 95% CI: 0.011, 0.301, p = 0.035, respectively; Table 2, Fig. 1). Because the lipid concentrations tend to be variable over time, we performed sensitivity analyses by using relative measures of LPCs, calculated as a ratio of the individual concentration over the sum of all LPCs concentration. Results for LPC 16:1 and 18:1 remained similar using the relative measures; lower baseline LPC 16:1 and faster rates of decline in LPC 16:1 and 18:1 over time were associated with a faster rate of decline in kPCr (B = − 0.192, 95% CI: − 0.352, − 0.033, p = 0.018; B = 0.227, 95% CI: 0.084, 0.370, p = 0.002; B = 0.17, 95% CI: 0.022, 0.318, p = 0.024, respectively).
Discussion
We previously reported that lower levels of specific plasma LPCs are cross-sectionally associated with lower mitochondrial function. Here, we extend our previous findings by demonstrating that baseline LPCs (16:1) and change in LPCs over time (16:1 and 18:1) are associated with a longitudinal change in skeletal muscle mitochondrial function over time in the same direction.
A novel finding in this longitudinal study is that we found that only selected LPC species, especially 16:1, and 18:1, were associated with the change in mitochondrial function, whereas both this and previous studies found more LPC species were cross-sectionally associated with mitochondrial function (including 16:0, 17:0, 18:0, 18:2, 20:3 in addition to 16:1 and 18:1). Longitudinal findings support the causal hypothesis because they track within-individual change over time and reflect a meaningful temporal sequence that cannot be fully captured by cross-sectional analysis. Associations with LPC C16:1 and 18:1 remained robust in analyses of the relative ratio measures. In addition, lower LPC C16:1 at baseline was also associated with subsequent change in skeletal muscle mitochondrial function. Evidence from animal studies has shown that LPC C16:1 (palmitoleic acid 16:1, n-7) is positively correlated with succinate-fueled state 3 mitochondrial respiration [1] and can enhance mitochondrial fatty acid oxidation, oxygen consumption, and ATP content in white adipocyte [6]. Our findings suggest that interventions that increase plasma levels of specific LPC classes may prevent the decline of mitochondrial function that is often observed with aging and chronic diseases. Dietary LPC 18:1 (oleic acid 18:1) can be efficiently incorporated into cardiolipin [35] and contribute to remodeling of the cardiolipin profile [24]. Some animal studies have shown that vitamin E increases LPC 16:0, which can have anti-inflammatory and anti-ROS properties [3, 14, 18, 20, 23], and that the intake of LPC 18:0 protects against obesity [12]. Recent data have shown that a 30-day medium-chain triglycerides intervention induced ketosis, increased concentrations of LPC 16:0, P-18:1, P-18:0, 20:2, and 22:5, and improved cognitive function in patients with mild to moderate Alzheimer’s disease who are not apolipoprotein E ɛ4 carriers [39]. Importantly, increases in LPC P-18:1 were associated with decreases in cognitive impairment scores after the intervention. These findings are supported by previous intervention showing that the ketogenic agent improved cognitive performance in patients with mild to moderate Alzheimer’s disease [13]. Several studies have shown that ketogenic diets can not only improve cognitive function but also affect other metabolic pathways, such as the increased activity of mitochondrial uncoupling proteins, decreased free radical production, improved ATP production in mitochondria, and improved mitochondrial membrane potential [29].
Data are emerging on the important roles of lipids and lipid metabolism in aging and age-related disease, such as Alzheimer’s disease (for review, see [16, 38]. Human intervention studies have shown that the intake of median chain triglycerides and eicosapentaenoic acid/docosahexaenoic acid (EPA/DHA) supplementation significantly increased certain plasma lysophosphatidylcholine concentrations [2, 39]. Future research is warranted to explore what dietary intake of lipids would change circulating lysophosphatidylcholines and thus affect lipid metabolism.
This study has several strengths. Repeated measures of metabolomics and skeletal muscle mitochondrial function allow us to investigate within-individual change over time. Assessment of in vivo skeletal muscle mitochondrial function using MR spectroscopy is state-of-the-art. Plasma samples were collected from fasting participants. In addition, examining both absolute concentration and relative ratio measures of LPCs confirmed the strength of the longitudinal association, specifically LPC 16:1 and 18:1. This study has some limitations. The study sample consists of well-characterized community-dwelling adults who tend to be healthier, more educated, and have higher socioeconomic status than the general adult population. The sample size is modest. Future studies with a larger sample and more repeated measures over a longer follow-up time are needed to confirm the findings of this study. Ultimately, the hypothesis that dietary intervention or supplementation that can increase the plasma level of LPCs may improve mitochondrial function should be tested in clinical trials.
References
Armstrong C, Thomas RH, Price ER, Guglielmo CG, Staples JF. Remodeling mitochondrial membranes during arousal from hibernation. Physiol Biochem Zool. 2011;84(4):438–49. https://doi.org/10.1086/660892.
Block RC, Duff R, Lawrence P, Kakinami L, Brenna JT, Shearer GC, Meednu N, Mousa S, Friedman A, Harris WS, Larson M, Georas S. The effects of EPA, DHA, and aspirin ingestion on plasma lysophospholipids and autotaxin. Prostaglandins Leukot Essent Fatty Acids. 2010;82(2–3):87–95. https://doi.org/10.1016/j.plefa.2009.12.005.
Choi J, Leonard SW, Kasper K, McDougall M, Stevens JF, Tanguay RL, Traber MG. Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. J Lipid Res. 2015;56(6):1182–90. https://doi.org/10.1194/jlr.M058941.
Choi S, Reiter DA, Shardell M, Simonsick EM, Studenski S, Spencer RG, Fishbein KW, Ferrucci L. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2016;71(12):1638–45. https://doi.org/10.1093/gerona/glw059.
Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE, Goodpaster BH. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–55. https://doi.org/10.1093/gerona/gls196.
Cruz MM, Lopes AB, Crisma AR, de Sa RCC, Kuwabara WMT, Curi R, de Andrade PBM, Alonso-Vale MIC. Palmitoleic acid (16:1n7) increases oxygen consumption, fatty acid oxidation and ATP content in white adipocytes. Lipids Health Dis. 2018;17(1):55. https://doi.org/10.1186/s12944-018-0710-z.
da Silva JF, Alves JV, Silva-Neto JA, Costa RM, Neves KB, Alves-Lopes R, Carmargo LL, Rios FJ, Montezano AC, Touyz RM, Tostes RC. Lysophosphatidylcholine induces oxidative stress in human endothelial cells via NOX5 activation - implications in atherosclerosis. Clin Sci (Lond). 2021;135(15):1845–58. https://doi.org/10.1042/CS20210468.
Drzazga A, Sowinska A, Koziolkiewicz M. Lysophosphatidylcholine and lysophosphatidylinosiol - novel promissing signaling molecules and their possible therapeutic activity. Acta Poloniae Pharmaceutica 2014;71(6), 887–99. <Go toISI>://WOS:000209899300002
Falabella M, Vernon HJ, Hanna MG, Claypool SM, Pitceathly RDS. Cardiolipin, mitochondria, and neurological disease. Trends Endocrinol Metab. 2021;32(4):224–37. https://doi.org/10.1016/j.tem.2021.01.006.
Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63(12):1416–9. https://doi.org/10.1093/gerona/63.12.1416.
Gonzalez-Freire M, Moaddel R, Sun K, Fabbri E, Zhang P, Khadeer M, Salem N Jr, Ferrucci L, Semba RD. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2019;74(1):62–7. https://doi.org/10.1093/gerona/gly100.
Han AR, Park HR, Kim GJ, Kim BR, Kim YR, Park HH, Park J, Jin CH, Kim JM, Kwon SJ, Kim JB, Cao S, Nam JW, Choi H. 18:0 Lyso PC derived by bioactivity-based molecular networking from lentil mutant lines and its effects on high-fat diet-induced obese mice. Molecules 2021;26(24). https://doi.org/10.3390/molecules26247547
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31. https://doi.org/10.1186/1743-7075-6-31.
Huang YH, Schafer-Elinder L, Wu R, Claesson HE, Frostegard J. Lysophosphatidylcholine (LPC) induces proinflammatory cytokines by a platelet-activating factor (PAF) receptor-dependent mechanism. Clin Exp Immunol. 1999;116(2):326–31. https://doi.org/10.1046/j.1365-2249.1999.00871.x.
Hung ND, Sok DE, Kim MR. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Inflamm Res. 2012;61(5):473–83. https://doi.org/10.1007/s00011-012-0434-x.
Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019;18(6): e13048. https://doi.org/10.1111/acel.13048.
Kim EA, Kim JA, Park MH, Jung SC, Suh SH, Pang MG, Kim YJ. Lysophosphatidylcholine induces endothelial cell injury by nitric oxide production through oxidative stress. J Matern Fetal Neonatal Med. 2009;22(4):325–31. https://doi.org/10.1080/14767050802556075.
Klingler C, Zhao X, Adhikary T, Li J, Xu G, Haring HU, Schleicher E, Lehmann R, Weigert C. Lysophosphatidylcholines activate PPARdelta and protect human skeletal muscle cells from lipotoxicity. Biochim Biophys Acta 2016; 1861(12 Pt A): 1980–1992. https://doi.org/10.1016/j.bbalip.2016.09.020
Knuplez E, Marsche G. An updated review of pro- and anti-inflammatory properties of plasma lysophosphatidylcholines in the vascular system. Int J Mol Sci 2020;21(12). https://doi.org/10.3390/ijms21124501
Lauritano C, Helland K, Riccio G, Andersen JH, Ianora A, Hansen EH. Lysophosphatidylcholines and chlorophyll-derived molecules from the diatom cylindrotheca closterium with anti-inflammatory activity. Mar Drugs 2020;18(3). https://doi.org/10.3390/md18030166
Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci 2019;20(5). https://doi.org/10.3390/ijms20051149
Li B, Tian S, Liu X, He C, Ding Z, Shan Y. Sulforaphane protected the injury of human vascular endothelial cell induced by LPC through up-regulating endogenous antioxidants and phase II enzymes. Food Funct. 2015;6(6):1984–91. https://doi.org/10.1039/c5fo00438a.
Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174(5):2981–9. https://doi.org/10.4049/jimmunol.174.5.2981.
McGee CD, Lieberman P, Greenwood CE. Dietary fatty acid composition induces comparable changes in cardiolipin fatty acid profile of heart and brain mitochondria. Lipids. 1996;31(6):611–6. https://doi.org/10.1007/BF02523831.
Meyerspeer M, Boesch C, Cameron D, Dezortova M, Forbes SC, Heerschap A, Jeneson JAL, Kan HE, Kent J, Layec G, Prompers JJ, Reyngoudt H, Sleigh A, Valkovic L, Kemp GJ, Experts’ Working Group on, P. M. R. S. o. S. M. (31) P magnetic resonance spectroscopy in skeletal muscle: experts’ consensus recommendations. NMR Biomed 2020;e4246. https://doi.org/10.1002/nbm.4246
Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31(4):269–86. https://doi.org/10.1016/s0010-4825(01)00006-3.
Nierenberg JL, He J, Li C, Gu X, Shi M, Razavi AC, Mi X, Li S, Bazzano LA, Anderson AH, He H, Chen W, Guralnik JM, Kinchen JM, Kelly TN. Serum metabolites associate with physical performance among middle-aged adults: evidence from the Bogalusa Heart Study. Aging (Albany NY) 2020; 12(12): 11914–41. https://doi.org/10.18632/aging.103362
Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta. 2014;1837(4):408–17. https://doi.org/10.1016/j.bbabio.2013.10.006.
Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab. 2008;28(1):1–16. https://doi.org/10.1038/sj.jcbfm.9600543.
Schilling T, Eder C. Sodium dependence of lysophosphatidylcholine-induced caspase-1 activity and reactive oxygen species generation. Immunobiology. 2011;216(1–2):118–25. https://doi.org/10.1016/j.imbio.2010.06.010.
Schlame M, Greenberg ML. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(1):3–7. https://doi.org/10.1016/j.bbalip.2016.08.010.
Semba RD, Zhang P, Adelnia F, Sun K, Gonzalez-Freire M, Salem N Jr, Brennan N, Spencer RG, Fishbein K, Khadeer M, Shardell M, Moaddel R, Ferrucci L. Low plasma lysophosphatidylcholines are associated with impaired mitochondrial oxidative capacity in adults in the Baltimore Longitudinal Study of Aging. Aging Cell. 2019;18(2): e12915. https://doi.org/10.1111/acel.12915.
Shock N, Greulich R, Andres R, Arrenberg D, Costa PT, Jr., Lakatta E, Tobin J. Normal human aging: the baltimore longitudinal study of aging. U.S. Government Printing Office, Washington, DC 20402, NIH Publication No 84–2450. 1984.
Stock C, Schilling T, Schwab A, Eder C. Lysophosphatidylcholine stimulates IL-1beta release from microglia via a P2X7 receptor-independent mechanism. J Immunol. 2006;177(12):8560–8. https://doi.org/10.4049/jimmunol.177.12.8560.
Ting HC, Chen LT, Chen JY, Huang YL, Xin RC, Chan JF, Hsu YH. Double bonds of unsaturated fatty acids differentially regulate mitochondrial cardiolipin remodeling. Lipids Health Dis. 2019;18(1):53. https://doi.org/10.1186/s12944-019-0990-y.
Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–7. https://doi.org/10.1021/pr800545q.
Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140(1):120–30. https://doi.org/10.1006/jmre.1999.1835.
Wlodarek D. The possibility of use of the ketogenic diet and medium chain triglycerides supplementation in the support therapy of Alzheimer disease. Curr Opin Clin Nutr Metab Care. 2021;24(4):385–91. https://doi.org/10.1097/MCO.0000000000000752.
Xu Q, Zhang Y, Zhang X, Liu L, Zhou B, Mo R, Li Y, Li H, Li F, Tao Y, Liu Y, Xue C. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer’s disease patients with APOE4(-/-): a double-blind, randomized, placebo-controlled crossover trial. Clin Nutr. 2020;39(7):2092–105. https://doi.org/10.1016/j.clnu.2019.10.017.
Yamaguchi Y, Zampino M, Moaddel R, Chen TK, Tian Q, Ferrucci L, Semba RD. Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging. Metabolomics. 2021;17(1):9. https://doi.org/10.1007/s11306-020-01762-3.
Zane AC, Reiter DA, Shardell M, Cameron D, Simonsick EM, Fishbein KW, Studenski SA, Spencer RG, Ferrucci L. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell. 2017;16(3):461–8. https://doi.org/10.1111/acel.12568.
Acknowledgements
This work was fully supported by the Intramural Research Program of the National Institute on Aging.
Author information
Authors and Affiliations
Contributions
QT developed the study concept and statistical analysis plan, analyzed, interpreted, and drafted the manuscript. BAM performed statistical analyses, interpreted, and drafted the manuscript. MZ critically evaluated the manuscript. LF designed the study and critically evaluated the manuscript. All authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tian, Q., Mitchell, B.A., Zampino, M. et al. Longitudinal associations between blood lysophosphatidylcholines and skeletal muscle mitochondrial function. GeroScience 44, 2213–2221 (2022). https://doi.org/10.1007/s11357-022-00548-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00548-w