Abstract
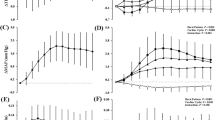
Higher aerobic fitness is independently associated with better cardiovascular health in older adults. The transduction of muscle sympathetic nerve activity (MSNA) into mean arterial pressure (MAP) responses provides important insight regarding beat-by-beat neural circulatory control. Aerobic fitness is negatively associated with peak MAP responses to spontaneous MSNA in young males. Whether this relationship exists in older adults is known. We tested the hypothesis that aerobic fitness was inversely related to sympathetic neurohemodynamic transduction and blood pressure variability (BPV) in older adults. Relative peak oxygen consumption (V̇O2peak, indirect calorimetry) was assessed in 22 older adults (13 males, 65 ± 5 years, 36.3 ± 11.5 ml/kg/min). Peroneal MSNA (microneurography) and arterial pressure (finger photoplethysmography) were recorded during ≥ 10-min of rest. BPV was assessed using the average real variability index. MAP was tracked for 12 cardiac cycles following heartbeats associated with MSNA bursts (i.e., peak ΔMAP). Peak ΔMAP responses (0.9 ± 0.6 mmHg) were negatively associated (all, P < 0.04) with resting burst frequency (30 ± 11 bursts/min; R = -0.47) and burst incidence (54 ± 22 bursts/100 heartbeats; R = -0.51), but positively associated with BPV (ρ = 0.47). V̇O2peak was inversely related to the pressor responses to spontaneous bursts (R = -0.47, P = 0.03) and BPV (ρ = -0.54, P = 0.01), positively related to burst incidence (R = 0.42, P = 0.05), but unrelated to MSNA burst frequency (P = 0.20). The V̇O2peak-BPV relationship remained after controlling for burst frequency, peak ΔMAP, age, and sex. Lower V̇O2peak was associated with augmented neurohemodynamic transduction and BPV in older adults. These negative hemodynamic outcomes highlight the importance of higher aerobic fitness with ageing for optimal cardiovascular health.



Similar content being viewed by others
References
McKinney J, Lithwick D, Morrison B, et al. The health benefits of physical activity and cardiorespiratory fitness. BCMJ. 2016;58:131–7.
Jackson AS, Sui X, Hébert JR, et al. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–7. https://doi.org/10.1001/archinternmed.2009.312.
Laukkanen JA, Zaccardi F, Khan H, et al. Long-term change in cardiorespiratory fitness and all-cause mortality. Mayo Clin Proc. 2016;91:1183–8. https://doi.org/10.1016/j.mayocp.2016.05.014.
Hart EC, Charkoudian N. Sympathetic neural mechanisms in human blood pressure regulation. Curr Hypertens Rep. 2011;13:237–43. https://doi.org/10.1007/s11906-011-0191-1.
Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Circ Physiol. 2000;278:H1205–10. https://doi.org/10.1152/ajpheart.2000.278.4.H1205.
Keir DA, Badrov MB, Tomlinson G, et al. Influence of sex and age on muscle sympathetic nerve activity of healthy normotensive adults. Hypertens (Dallas, Tex 1979). 2020; 76:997–1005. https://doi.org/10.1161/HYPERTENSIONAHA.120.15208
Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–5. https://doi.org/10.1007/BF01826234.
Hart ECJ, Charkoudian N. Sympathetic neural regulation of blood pressure: Influences of sex and aging. Physiology. 2014;29:8–15. https://doi.org/10.1152/physiol.00031.2013.
Ng AV, Callister R, Johnson DG, Seals DR. Endurance exercise training is associated with elevated basal sympathetic nerve activity in healthy older humans. J Appl Physiol. 1994;77:1366–74. https://doi.org/10.1152/jappl.1994.77.3.1366.
Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587:2049–57. https://doi.org/10.1113/jphysiol.2009.170134.
Baker SE, Limberg JK, Scruggs ZM, et al. Greater influence of aerobic fitness on autonomic support of blood pressure in young women than in older women. Hypertension. 2020;75:1497–504. https://doi.org/10.1161/HYPERTENSIONAHA.119.14042.
Fairfax ST, Holwerda SW, Credeur DP, et al. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol. 2013;591:3637–49. https://doi.org/10.1113/jphysiol.2013.250894.
Vranish JR, Holwerda SW, Young BE, et al. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension. 2018;71:192–8. https://doi.org/10.1161/HYPERTENSIONAHA.117.10229.
Babcock MC, Robinson AT, Migdal KU, et al. Reducing dietary sodium to 1000 mg per day reduces neurovascular transduction without stimulating sympathetic outflow. Hypertension. 2019;73:587–93. https://doi.org/10.1161/HYPERTENSIONAHA.118.12074.
Hissen SL, Taylor CE. Sex differences in vascular transduction of sympathetic nerve activity. Clin Auton Res. 2020;30:381–92. https://doi.org/10.1007/s10286-020-00722-0.
O’Brien MW, Ramsay D, Johnston W, Kimmerly DS Aerobic fitness and sympathetic responses to spontaneous muscle sympathetic nerve activity in young males. Clin Auton Res In Press. https://doi.org/10.1007/s10286-020-00734-w
Young BE, Greaney JL, Keller DM, Fadel PJ. Sympathetic transduction in humans: recent advances and methodological considerations. Am J Physiol Circ Physiol ajpheart. 2021; 00926.2020. https://doi.org/10.1152/ajpheart.00926.2020
Coovadia Y, Adler TE, Steinback CD, et al. Sex differences in dynamic blood pressure regulation: beat-by-beat responses to muscle sympathetic nerve activity. Am J Physiol Circ Physiol. 2020;319:H531–8. https://doi.org/10.1152/ajpheart.00245.2020.
Steinback CD, Fraser GM, Usselman CW, et al. Blunted sympathetic neurovascular transduction during normotensive pregnancy. J Physiol. 2019;597:3687–96. https://doi.org/10.1113/JP277714.
Young BE, Holwerda SW, Vranish JR, et al. Sympathetic transduction in type 2 diabetes mellitus. Hypertension. 2019;74:201–7. https://doi.org/10.1161/HYPERTENSIONAHA.119.12928.
Vianna LC, Hart EC, Fairfax ST, et al. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Circ Physiol. 2012;302:H2419–27. https://doi.org/10.1152/ajpheart.01105.2011.
Berthelsen LF, Fraser GM, Simpson LL, et al. Highs and lows of sympathetic neurocardiovascular transduction: influence of altitude acclimatization and adaptation. Am J Physiol Circ Physiol. 2020;319:H1240–52. https://doi.org/10.1152/ajpheart.00364.2020.
Watso JC, Babcock MC, Migdal KU, et al The relation between habitual physical activity and sympathetic vascular transduction in healthy young adults. Clin Auton Res In Press. https://doi.org/10.1007/s10286-021-00770-0
Robinson AT, Babcock MC, Watso JC, et al. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: sex differences in healthy young adults. Am J Physiol Integr Comp Physiol. 2019;316:R463–71. https://doi.org/10.1152/ajpregu.00305.2018.
Wei FF, Li Y, Zhang L, et al. Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated chinese. Hypertension. 2014;63:790–6. https://doi.org/10.1161/HYPERTENSIONAHA.113.02681.
Hissen SL, Macefield VG, Brown R, Taylor CE. Sympathetic baroreflex sensitivity is inversely related to vascular transduction in men but not women. Am J Physiol Circ Physiol. 2019;317:H1203–9. https://doi.org/10.1152/ajpheart.00501.2019.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. https://doi.org/10.3758/BRM.41.4.1149.
Briant LJB, Burchell AE, Ratcliffe LEK, et al. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol. 2016;594:4753–68. https://doi.org/10.1113/JP272167.
Thijssen DHJ, Bruno RM, van Mil ACCM, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–47. https://doi.org/10.1093/eurheartj/ehz350.
Borg G. Psychophysical basis of perceived exertion. Med Sci Sport Exerc. 1982;14:377–81. https://doi.org/10.1249/00005768-198205000-00012.
Hart EC, Head GA, Carter JR, et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Circ Physiol. 2017;312:H1031–51. https://doi.org/10.1152/ajpheart.00703.2016.
Kimmerly DS, O’Leary DD, Shoemaker JK. Test–retest repeatability of muscle sympathetic nerve activity: influence of data analysis and head-up tilt. Auton Neurosci. 2004;114:61–71. https://doi.org/10.1016/j.autneu.2004.06.005.
O’Brien MW, Petterson JL, Kimmerly DS. An open-source program to analyze spontaneous sympathetic neurohemodynamic transduction. J NeurophysiolIn Press. 2021; https://doi.org/10.1152/jn.00002.2021
Mena L, Pintos S, Queipo NV, et al. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–11. https://doi.org/10.1097/01.hjh.0000160205.81652.5a.
Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24-Hour blood pressure variability assessed by average real variability: a systematic review and meta-analysis. J Am Heart Assoc. 2017; 6.https://doi.org/10.1161/JAHA.117.006895
Young BE, Kaur J, Vranish JR, et al. Augmented resting beat-to-beat blood pressure variability in young, healthy, non-Hispanic black men. Exp Physiol. 2020;105:1102–10. https://doi.org/10.1113/EP088535.
Notarius CF, Murai H, Morris BL, Floras JS. Effect of fitness on reflex sympathetic neurovascular transduction in middle-age men. Med Sci Sport Exerc. 2012;44:232–7. https://doi.org/10.1249/MSS.0b013e31822a68a5.
Billman GE, Cagnoli KL, Csepe T, et al. Exercise training-induced bradycardia: evidence for enhanced parasympathetic regulation without changes in intrinsic sinoatrial node function. J Appl Physiol. 2015;118:1344–55. https://doi.org/10.1152/japplphysiol.01111.2014.
Poehlman ET, Danforth E. Endurance training increases metabolic rate and norepinephrine appearance rate in older individuals. Am J Physiol Metab. 1991;261:E233–9. https://doi.org/10.1152/ajpendo.1991.261.2.E233.
Esler MD, Hasking GJ, Willett IR, et al. Noradrenaline release and sympathetic nervous system activity. J Hypertens. 1985;3:117–29. https://doi.org/10.1097/00004872-198504000-00003.
Ninomiya I, Malpas SC, Matsukawa K, et al. The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre- and postganglionic fibers in anesthetized cats. J Auton Nerv Syst. 1993;45:139–47. https://doi.org/10.1016/0165-1838(93)90125-E.
Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579:115–25. https://doi.org/10.1113/jphysiol.2006.120055.
O’Brien MW, Ramsay D, Johnston W, Kimmerly D. The association between habitual posture and intensity-related physical activity with sympathetic neurohemodynamic transduction in young males.Clin Auton Res In Press. 2021; https://doi.org/10.1007/s10286-021-00802-9
Funding
Canadian Foundation for Innovation: Leader’s Opportunity Fund (DSK), Faculty of Health Professions Research Development (DSK), and Nova Scotia Health Research Foundation (NSHRF) Development/Innovation (DSK) grants. MWO was supported by a Heart & Stroke BrightRed Scholarship, Nova Scotia Graduate Scholarship, a Research Nova Scotia—Scotia Scholars Award, a Killam PreDoctoral Scholarship, and a Fredrick Banting and Charles Best CIHR Doctoral Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11357_2021_389_MOESM1_ESM.pdf
Supplemental Fig. 1 Beat-by-beat total peripheral resistance (TPR; top panel) and cardiac output (CO; bottom panel) responses following cardiac cycles associated with (bursts), or absent of (non-bursts), muscle sympathetic nerve activity. Data presented separately for bursts (filled circles) and non-bursts (white circles). The broken black line represents no changes in TPR or CO from cardiac cycle zero. Data presented as means ± standard deviations. Data were analyzed using a Burst Presence (2) × Cardiac cycle (12) repeated measure analysis of variance with Bonferroni post hoc testing. *P < 0.05 between bursts and non-bursts (PDF 268 KB)
11357_2021_389_MOESM2_ESM.pdf
Supplemental Fig. 2 The relationship between systolic blood pressure (SBP; left panels) and diastolic blood pressure (DBP; right panels) average real variability (ARV) with aerobic fitness (relative V̇O2peak; top panels), the peak pressor responses following bursts of MSNA (middle panels; peak ΔMAP) and following cardiac cycles absent of bursts (bottom panels; nadir ΔMAP; white circles). Males and females are presented as circles and triangles, respectively. Relationships were determined via Pearson correlations or non-parametric Spearman’s rank-order correlations (PDF 268 KB)
About this article
Cite this article
O’Brien, M.W., Ramsay, D.J., O’Neill, C.D. et al. Aerobic fitness is inversely associated with neurohemodynamic transduction and blood pressure variability in older adults. GeroScience 43, 2737–2748 (2021). https://doi.org/10.1007/s11357-021-00389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-021-00389-z