Abstract
In this study, PDMS incorporated with SWCNTs have been fabricated via solution casting method for industrial applications and characterized by the analyses of SEM, FTIR, TGA, AFM, and MST. The modified membranes were further analyzed for CO2, O2, and N2 gas permeability. The strategic membranes have five different weight ratios (0.013, 0.025, 0.038, 0.050, 0.063) compared to neat PDMS membranes. The even distribution of SWCNTs in PDMS provided results that showed improvement in thermal stability. However, mechanical strength has been weakened with increased concentration of nanofiller because of the increase in the number of SWCNTs by increases that imperfections become more severe. The designed polymeric membranes with good thermal stability and adequate mechanical strength can be used for the selectivity and permeability of CO2, O2, and N2 gases. The effect of the PDMS-SWCNTs on gas permeability has been studied. 0.063 wt.% SWCNTs presented the maximum permeability of CO2 gas while maximum O2 and N2 gas permeability have been obtained by 0.013 wt.% SWCNTs. The ideal selectivity of mixed (50:50) gas conditions has been tested. The maximum CO2/N2 ideal selectivity was obtained by 0.050 and 0.063 wt.% SWCNTs while maximum O2/N2 ideal selectivity obtained by 0.050 wt.% SWCNTs. Therefore, the fabrication of this novel SWCNTs-PDMS membrane may lead to separating the industrial exhaust and be used as a potential membrane for environmental remediation in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the gas separation process, membrane technology plays a very influential role. A wide range of polymeric membranes is used in many industrial and commercial applications due to their excellent features. These polymeric gas separation membranes are very compact in size, have less capital cost, and have better modular configuration, leading to less power consumption and decreasing production costs (Quan et al. 2017). The carbon emission is very low from biogas compared to coal heating and liquefied petroleum (Hossain and Morni 2020). The environmental impact and energy consumption cost are significant challenges due to the increment in energy demand and mitigation of global climate warming. Many commercial and industrial applications use polymeric membranes to overcome these climate change impacts (Mubashir et al. 2021; Viannie et al. 2021).
Polymeric membranes have been applied in environmental applications like water purification and pre and post gas combustion due to their significant features. These membranes are also used in drug delivery devices and the separation of various gas mixtures. Polymeric membranes have great importance in CO2 separation from the air due to their energy-saving cost and operational process simplicity, which have become an excellent curiosity for researchers (Mubashir et al. 2018). The polymeric membranes have weak interactions between macromolecules, or the existence of a statistically distributed free volume, allowing gasses to permeate through the polymer. Hybrid polymeric membranes have increased permeability due to the increased contribution of diffusion components (Baker 2000; Iqbal et al. 2021; Viannie et al. 2021).
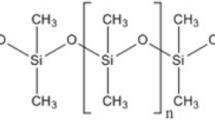
One of the most promising polymers is polydimethylsiloxane (PDMS). It has good chemical and thermal stability, biocompatibility, ease of use, chemical inertia, hyperplastic characteristics, and gas permeability at a low cost. Thus, PDMS has been used in several fields and systems (microfluidics/nanofluidics, electronic components, membranes for filtering and pervaporation, sensors, thermal devices, coatings, and others). With compromised mechanical properties, the tailored PDMS composites with nano-reinforcements can improve the properties of PDMS. The PDMS polymeric composite membranes are used in many gas separation applications and have significant importance due to their high permeability and flexibility in nature. Different inorganic, polymeric, and hybrid membrane materials are developed, and their challenges are studied (Javaid 2005; Pan et al. 2022). The block copolymer is synthesized by atom transfer radical polymerization technique for thermodynamic study. The cylindrical micelles of copolymers were confirmed by Flory–Huggins, and solubility parameters along the phase-separated X-ray analyze morphology. The fibrillar morphology of the blend membrane had good permeation and selectivity (Fishlock et al. 2018, Semsarzadeh and Ghahramani 2015, Shahapurkar et al. 2021). A freely suspended PDMS membrane is synthesized in situ. The amphiphilic properties of PDMS precursors make them suitable for water/air treatment (Bilotkach and Lee 2008). Spectral and thermal studies of PDMS with increasing concentrations of cross-linkers and nano-reinforcements were used to characterize gas selectivity applications (Alvaro and Roy 2005, Jadhav et al. 2020; Kammermeyer 1957). These membranes are advantageous for separation because of their permeability, low cost, good mechanical stability, ease of processability, and reasonably good selectivity (Adrees et al. 2019). The earlier study re-examined the relationship between CO2/CH4 selectivity and CO2 permeability. This study reported a modest shift in polymer-bound positions for gas pairs since 1991 (Jadhav et al. 2020; Robeson 2008).
The polymeric membranes have significantly less energy and are separated at a molecular level. It is observed that the separation properties of the membrane have improved with the addition of multi-walled carbon nanotubes (MWCNTs) (Mazari et al. 2021; Sanip et al. 2011). MWCNTs/PDMS composites were synthesized as membranes to evaluate their gas separation properties for the separation of H2 from CH4 gas species (Nour et al. 2013, Rutnakornpituk and Ngamdee 2006). PDMS membranes with varying concentrations of multi-walled carbon nanotubes were prepared to evaluate their gas separation performances of CO2, CH4, N2, and O2 (Berean et al. 2014; Mubashir et al. 2016; Silva et al. 2017). The evaluation of membranes using ZIF-62 metal–organic framework nano-hybrid dots was discussed for environmental remediation (Mubashir et al. 2022). The molecular-scale fabrication technique is used to fabricate unique CNT-ZIF-8-PDMS composite membranes with enhanced gas separation performance, mainly focusing on CO2 capturing (CO2/N2, CO2/H2) (Ashtiani et al. 2021; Reijerkerk et al. 2010). In the inert pyrolysis process, the polyimide siloxane was converted into the carbon-rich phase of the imide domains. Later, molecular sieving capabilities were checked for minor gasses O2/N2, CO2/N2, H2/N2, and He/N2. In polyimide siloxane, the siloxane domains are further converted into the silica-rich phase to enhance gas pathways (Gao et al. 2022; Park et al. 2004).
The effects of the SWCNTs upon exposure to high-energy protons, the stopping ranges for PDMS/SWCNT, and pure PDMS were simulated, and the results were compared to those obtained from the experiments (Li et al. 2013). An earlier experimental study (Kim et al. 2006) discussed the nanocomposite membranes consisting of single-walled carbon nanotubes embedded in a poly(imide siloxane) copolymer, evaluated their transport properties, and suggested that the single-walled carbon nanotubes (SWCNTs) offer an attractive additive for universally enhancing the gas permeability of polymers. The promising features of SWCNT/PIS membranes for augmentation of gas permeability urge us to study the processes for further development of gas permeability/selectivity. Therefore, this study emphasized an experimental attempt to determine the influence of SWCNTs for stimulating the properties of PDMS to explore the possibility of a higher efficient membrane compared to existing literature. Hence, the novelty of this study lies in exploring SWCNTs on PDMS membranes for CO2, O2, and N2 gas selectivity and permeability that may lead to the high potential of this modified membrane in industrial applications to separate exhaust waste more effectively in future research.
The main objective of this experimental study is to investigate the efficiency of gas separation and permeability and purify the polluted gas from industries which may lead to protecting humans and the ecosystem from harmful and toxic elements in exhausted gasses. Novel polymeric membranes of SWCNTs-PDMS are fabricated in this study using the solution casting method with varying concentrations of nano-reinforcements carbon-based SWCNTs to achieve this goal. The solution casting method is used to synthesize SWCNTs-PDMS polymeric membranes for the spectral, thermal, and mechanical efficacy of the resultant. The spectral, thermal stability, and mechanical strength analyses have been performed for enhanced gas permeation at ambient temperature with 20 psi pressure for possible future research on permeability for CO2, O2, and N2 in industrial applications and environmental remediation.
Materials and methods
Materials
For the fabrication of gas membranes, toluene, used as a solvent for membrane fabrication, was purchased from SIGMA-Aldrich, UK. Polydimethylsiloxane (PDMS) (Elastosil LR 3003/50 A/B) elastomeric material was purchased from Wacker Chemie Company, Germany. Single-walled carbon nanotubes (SWCNTs) are obtained from United States Research Nanomaterials Inc., USA. All these required chemicals were analytical grade, and purity was 97–99%.
Fabrication of pure PDMS membrane
A pure PDMS solution was prepared by solution casting method with the addition of 2 g of elastomeric material Elastosil LR 3003/50-A in a toluene solvent of 30 mL at the temperature of 50 °C with constant stirring for 1.5 h. After completely dissolving in the toluene solvent, 2 g of part Elastosil LR 3003/50 B was added at the same temperature of 50 °C for 2 h, stirring the mixture constantly. The prepared viscous solution was then sonicated for 30 min for trapped air removal. The homogenized viscous solution proceeded to fabricate designed polymeric membranes within 4 h. Different ratio % ranges of SWCNTs 0.013, 0.025, 0.038, 0.050, and 0.063 are named A, B, C, D, and E, respectively, fabricated with the same procedure. The viscous solution was slowly poured into the petri dish. Extensive care was taken during pouring to obtain uniform thickness and to avoid air bubbles. The casted membrane petri dish was placed into the oven at 50 °C for 30 min. Then, the temperature was increased to 60 °C for 30 min, after raising the temperature with 10 °C intervals of 30 min up to 120 °C. This process was demonstrated under the controlled evaporation rate, which leads to precipitation. This slow heating process is used to cure the designed membranes without air bubbles trapping the surface of the membrane (Silva et al. 2017). Thus, the resultant PDMS/SWCNTs reinforced membranes were fabricated, and the composite was removed from the petri dish with a sharp knife. The thickness of all prepared membranes was analyzed using a SATRA gauge. These fabricated reinforced nanocomposite membranes have been used to analyze gas permeation performance. Figure 1 presents the overall technique for PDMS/SWCNTs membrane preparation within 4.5 h.
Characterization
Gas permeation process
The fundamental parameters of prepared nanocomposite membranes were used for gas separation membranes, elaborated below.
-
1-
Solubility
-
2-
Permeability
-
3-
Diffusion
The permeation was measured by various gasses. But in this paper, CO2, O2, and N2 were used to analyze the gas permeation.
Permeation measurement process
The gas permeation was measured at room temperature (25 ± 1 °C) with single gas using the constant-volume variable-pressure method in the test chamber (Mahurin et al. 2010). Figure 2 presents the schematic diagram of the gas permeation unit.
The permeation can be measured by below two processes.
-
1-
Variable-pressure and constant-volume technique
-
2-
Variable-volume and constant-pressure technique
Gas permeation tests consist of fabricated membranes mounted on brass disks of 47 mm and have a central hole of 10 mm. The membrane was well-settled and tight in the module. The capillary tube was also adjusted in the module. Then, the flexible pipe was connected with a gas cylinder to measure the permeation. Then, membrane airtightness was done firmly and sealed with epoxy to keep its edges tight. The filter paper was used for mechanical strength, and gas resistance was neglected. All set parameters were checked. Gas was set free from the cylinder by opening the regulator. Then, the required pressure was adjusted to study the permeation. After gas flow, liquid volume in the capillary and time of output permeate was measured to calculate permeation.
The permeation is calculated using Eq. 1. The permeation of other gasses was also measured using the same formula.
where Q = flow rate, L = membrane thickness, A = area, and P = pressure difference.
Ideal selectivity
Equation 2 was used to calculate the ideal selectivity α of membranes.
where ideal selectivity is αA/B; gas A and gas B permeability is denoted by PA and PB, respectively.
Scanning electron microscopy (SEM)
The SEM was used to analyze the morphology and cross-sectional view of the prepared membranes. The electron beam of required energy was generated from an electron gun and then was focused on the sample by electronegative lenses. The electron beam was scanned on the rectangular-shaped sample. The secondary electrons revealed surface morphology. We got different greyscale images by variation in the number of electrons and electron speed reflected from a different place of the sample (Cazaux 2005). The SEM machine (JSM 6409A, JEOL, Japan) was used, and the sample was gold sputtered.
Atomic force microscopy (AFM)
In tapping mode, membrane surface roughness was analyzed using a Multimode AFM (Veeco Metrology Group, Santa Barbara, Canada).
Fourier transform infrared spectroscopy (FTIR)
The FTIR was used to analyze the functional groups attached to the polymer or material. The spectrometer in the infrared region can measure the absorbance of transmittance. The qualitative structure of the polymer is studied by wavelength/intensity curves of FTIR (Nowakowski et al. 2008). Fourier transform infrared analysis of membranes, Happ-Genzel instrument detector type DTGS having a resolution of 16, wavenumber at 4000–650 cm−1, and background scans are 96 were used.
Thermogravimetric analysis (TGA)
TGA was performed to measure the thermal stability of the membrane by using the basic concept of mass loss or decomposition of polymer concerning temperature and time. The sample was exposed to the crucible holder with a microbalance made from platinum, aluminum, quartz, or alumina, which uniformly transferred the heat to the sample. The Perkin Elmer (Diamond 100 TG/DTA, Japan) was used to study the sample. Nitrogen flow was maintained to remove all the corrosive gas at 15 mL·min−1, which may take part in the degradation process (Adnan Ahmad et al. 2017, T. Dollase and Spiess 2003).
Mechanical strength testing
The mechanical testing was performed to analyze the membrane’s tensile strength, evaluated by CRE-type tester SATRA STM 466, England. The BS EN ISO, 3376–2011 test method followed a 50 mm·min−1 extension speed. The maximum force was studied under the controlled environment of 23 ± 2 °C and 45 ± 5% relative humidity. The maximum fractured force was calculated by dividing the cross-sectional area of the sample by its width.
Results and discussion
Morphology analysis
SEM was used to investigate the efficient permeability of SWCNTs reinforced PDMS membrane (Hajili et al. 2022). The SWCNTs’ surface morphology, uniform dispersion, and porosity are examined to discover the profound reality. The morphological representation of the SEM micrograph is described in Fig. 3 with SWCNTs enhancement regarding concentration. The sample C3, loaded with 0.015 g depicted a smooth surface with no crack, and filler (SWCNTs) was well distributed on the surface compared to other samples. The obtained results also agreed with Afzal et al. (2016) on the effect of the distribution of carbon nanotubes. The D4 sample with SWCNTs concentration showed agglomeration and non-homogeneity of filler particles, as shown in Fig. 3. It demonstrates that the filler is not well instilled in the backbone of PDMS membranes compared to C3, which shows the smooth and even distribution of filler into the membrane structure aligned with the existing literature (Okolo et al. 2020).
Surface topography
The atomic force microscopy (AFM) images and surface roughness of the fabricated membranes are shown in Figs. 4 and 5, respectively. Figure 5 presents that the surface roughness on A1, B2, C3, D4, and E5 remains in similar range of ⁓30 nm. Therefore, it is clear from the AFM images and surface roughness that the surface is smooth, and roughness did not fluctuate with the loading of nano-reinforcements. Lower surface roughness indicates higher possibility of gas absorption and lesser fouling on the membrane surface (Iqbal et al. 2018). The results of AFM and roughness analyses are in agreement with SEM micrograph analysis. Therefore, all the nano-reinforced polymeric membranes present gas absorption capacity.
Structural analysis
FTIR is employed to investigate the functional group of the fabricated membranes. The IR transmittance spectrum of PDMS-reinforced SWCNTs membrane has been depicted in Fig. 6. Based on the FTIR result, the associated CH3 peaks were observed at 1415–1260 cm−1. In contrast, the Si–O-Si stretching group was found to range (from 1000 to 1100 cm−1), confirmed by the reported literature (Sagar et al. 2015). A lower wavenumber of 930 cm−1 appeared with the increased concentration of SWCNTs. This effect can be seen in other materials having carbon. At 835–855 cm−1 and 785–815 cm−1, Si–C and Si(CH3)2 peaks have been observed in the FTIR micrograph (Jamshaid et al. 2017).
Thermal analysis
TGA study was executed to observe the thermal stability of membranes using Perkin Elmer with the rate of 100 °C·min−1. Figure 7 presents the thermal decomposition. The thermogram was achieved by heating in cycles at 300–700 °C with an N2 gas flow rate of 15 mL·min−1. This gas eliminates all destructive gasses involved in membrane degradation (Iqbal et al. 2018).
Figure 7 depicts that until the 300 °C-onset point, the evaporation of the oxygen-related functional group occurred functionally. Five different compositions of reinforced SWCNTs were fabricated and examined their thermal degradation up to 500 °C. This decomposition took place due to the degradation of the polymer backbone. Based on the thermal profile in Fig. 7, no substantial changes were observed after the offset point at 700 °C. The thermal stability increased as SWCNTs concentration increased in the prepared membrane samples. Still, it showed an adverse effect when it crossed the SWCNTs optimum level, which acted as contamination in the membrane structure (Iqbal et al. 2013). The adverse effect occurred due to contamination depicted in the above TGA graph results. The weight loss % at equilibrium is observed as ⁓45–86% for all fabricated membranes.
Mechanical strength analysis
The SATRA tensile tester STM-566 was used to analyze the breaking strength of the developed membrane. BS EN ISO 3376 test method with jaws separation 50 mm·min−1 was used for this purpose. ISO-3376–2011 Modified test method has been used for measuring tensile strength and elongation. CRE-type SATRA STM 566 machine was used for this purpose. Comparison of tensile strength and elongation at break of PURE and SWCNTS-filled membrane were presented in Figs. 8 and 9, respectively.
It was observed that the mechanical features of PDMS membranes were enhanced in the host polymers by increasing the concentration of SWCNTs. But this behavior was not long-lasting as after a specific point, mechanical features get decreased. This behavior was observed due to the excess loading of nanofillers of SWCNTs. This excess was behaving like impurities in the backbone of the PDMS membrane’s structure, which finally tends to the failure of the backbone. This behavior was the same for elongation at break. Hence, sample C3 presented the maximum mechanical characteristics in terms of elongation strength (Berber and Hafez 2016).
Gas performance evaluation
The PDMS elastomeric material was selected to evaluate gas performance due to its economic cost and good mechanical strength (Robeson 1991). The CNTs, graphene, and zeolite were used to study the gas separation performance (Duval et al.1994). Still, we have a lot of room to investigate the peculiar properties of PDMS membranes (Liu and Kulprathipanja 2010). In this study, fabricated PDMS membranes were tested to check gas permeability by high-performance permeability tester CSI-135. The CO2, N2, and O2 gas permeability of the synthesized membrane was measured at ambient temperature (25 °C) at 20 psi pressure. Figure 10 shows the permeability of gasses with pure PDMS.
Permeation/ideal selectivity results at 25 °C
The gas permeation and ideal selectivity of CO2, O2, and N2 results are denoted in Table 1 at room temperature and graphically represented in Figs. 11 and 12.
As depicted in Table 1, Figs. 11 and 12, it has been observed that by increasing nanofillers in PDMS membranes, the permeability of CO2, O2, and N2 decreases, and ideal selectivity gets enhanced (Adrees et al. 2019). The decreasing trend from the first sample was that by increasing the concentration of SWCNTs, the permeability of the B2 and C3 samples was getting down and then abruptly increased. The solution diffusion model interpreted in terms of solubility and diffusivity of gas molecules in individual polymers explains membrane permeability and ideal selectivity. The graphical representation shows the inverse relationship of gas permeability and SWCNTs as the increasing concentration of SWCNTs permeability decreases. Permeability was reduced because SWCNTs nanofiller have high chain compactness, low segmental motion, and small free volume. It tends to decrease chain mobility and diffusivity of gas molecules in nanofiller-incorporated membranes due to these properties of SWCNTs. The observation declined permeability by enhancing the content of SWCNTs in synthesized membranes.
Conclusions
PDMS/SWCNTs polymeric composite membranes were fabricated using the thermally induced phase separation process, varying the SWCNTs nano-reinforced filler concentration ratio. The resultant membranes had excellent thermal stability with optimized mechanical for gas selectivity and permeation performance at ambient temperature with 20 psi pressure for CO2, O2, and N2, respectively. The 0.063 wt.% SWCNTs membrane (E5) was manifested as the best performing membrane for CO2 gas permeability and CO2/N2 idea selectivity, 0.013 wt.% SWCNTs membrane (A1) was the best for O2 and N2 permeability and 0.050 wt.% SWCNTs membrane (D4) mixed gas O2/N2 selectivity. By increasing the concentration of the nanofiller of SWCNTs in PDMS, the permeability tends to decrease, and then after a particular value of nanofiller incorporation, permeability gets enhanced. The increase of pore size owing to the higher concentration of nanofiller in host polymeric materials may act as an impurity that led to pore generation at a pressure, which is the reason behind the above behavior. Selectivity gave the same outcomes through the fabricated membranes. Thermal stability is enhanced with the incorporation of SWCNTs nanofiller.
On the other hand, the mechanical characteristics follow the same trend as permeability due to the nanofiller’s overloading. The 0.038 wt.% reinforced SWCNTs give the maximum tensile force, 37.350 N, tensile strength, 8.893 N·mm−2, and elongation at break, 293.149 mm, while 0.013 wt.% has low tensile force, strength, and elongation at break. Therefore, the outcomes of this designed novel research revealed that SWCNT/PDMS polymeric nanocomposite membranes are compatible and hence facilitate the development of a homogeneous dense film structure with excellent potential for efficient membranes suitable for targeted gas separation with high commercial and environmental applications.
Data availability
The data that support the findings of this study are available from the corresponding author, Nazia Hossain, upon reasonable request.
Abbreviations
- PDMS:
-
Polydimethylsiloxane
- SWCNTs:
-
Single-walled carbon nanotubes
- TGA:
-
Thermogravimetric analysis
- CO2 :
-
Carbon dioxide gas
- O2 :
-
Oxygen gas
- N2 :
-
Nitrogen gas
- CH4 :
-
Methane
- SEM:
-
Scanning electron microscopy
- AFM:
-
Atomic force microscopy
- FTIR:
-
Fourier transform infrared analysis
- MST:
-
Mechanical stability testing
References
Adnan Ahmad FJ, Adrees M, Iqbal SS, Sabir A, Riaz T, Zaheer H, Islam A, Jamil T (2017) Novel polyurethane/polyvinyl chloride-co-vinyl acetate crosslinked membrane for reverse osmosis (RO). Desalination 420:136–144
Adrees M, Iqbal SS, Ahmad A, Jamshaid F, Haider B, Khan MH, Khan R, Butt MTZ, Bahadar A (2019) Characterization of novel polydimethylsiloxane (PDMS) and copolymer polyvinyl chloride-co-vinyl acetate (PVCA) enhanced polymer blend membranes for CO2 separation. Polym Testing 80:106163
Afzal A, Rafique MS, Iqbal N, Qaiser AA, Anwar AW, Iqbal SS (2016) Synergistic effect of functionalized nanokaolin decorated MWCNTs on the performance of cellulose acetate (CA) membranes spectacular. Nanomaterials 6:79
Alvaro Mata AJF, Roy AS (2005) Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Springer Science + Business Media, Inc. Manuf The Netherlands 7:281–293
Ashtiani S, Sofer Z, Průša F, Friess K (2021) Molecular-level fabrication of highly selective composite ZIF-8-CNT-PDMS membranes for effective CO2/N2, CO2/H2 and olefin/paraffin separations. Sep Purif Technol 274:119003
Baker RW (2000): Baker, Richard W. -- Kirk-Othmer encyclopedia of chemical technology __ membrane technology.pdf>. Wiley Online Library,
Berber M, Hafez IH (2016) Carbon nanotubes: current progress of their polymer composites
Berean K, Ou JZ, Nour M, Latham K, McSweeney C, Paull D, Halim A, Kentish S, Doherty CM, Hill AJ, Kalantar-zadeh K (2014) The effect of crosslinking temperature on the permeability of PDMS membranes: evidence of extraordinary CO2 and CH4 gas permeation. Sep Purif Technol 122:96–104
Bilotkach K, Lee AAP (2008) Fabrication of PDMS membranes with aqueous molds for microfluidic systems. Twelfth international conference on miniaturized systems for chemistry and life sciences and University of California, Irvine, USA 1483
Cazaux J (2005) <Recent developments and new strategies in scanning.pdf>. J Microsc 217:16–35
Dollase T, Wilhelm M, Spiess HW (2003) Effect of interfaces on the crystallization behavior of PDMS. Interface Sci 11:199–209
Fishlock SJ, Pu SH, Bhattacharya G, Han Y, McLaughlin J, McBride JW, Chong HMH, O’Shea SJ (2018) Micromachined nanocrystalline graphite membranes for gas separation. Carbon 138:125–133
Gao C-M, Cai J-N, Liu S-H, Ji S-F, Xing Y-Q, Chen J-C, Chen H-Y, Zou P, Wu J-J, Wu T-Y (2022) Superhydrophilic polyethersulfone (PES) membranes with high scale inhibition properties obtained through bionic mineralization and RTIPS. Environ Res 204:112177
Hajili E, Suo Z, Sugawara A, Asoh T-A, Uyama H (2022) Fabrication of chitin monoliths with controllable morphology by thermally induced phase separation of chemically modified chitin. Carbohyd Polym 275:118680
Hossain N, Morni NAH (2020) Co-pelletization of microalgae-sewage sludge blend with sub-bituminous coal as solid fuel feedstock. BioEnergy Research 13:618–629
Iqbal N, Khan MB, Sagar S, Maqsood A (2013) Fabrication and characterization of multiwalled carbon nanotubes/silicone rubber composites. J Appl Polym Sci 128:2439–2446
Iqbal SS, Inam F, Bahadar A, Bashir MA, Hassan F, Khan MB, Khan ZM, Jamil T (2018) Ablation, thermal stability/transport/phase transition study of carbon nanofiber-reinforced elastomeric nanocomposites. J Therm Anal Calorim 131:2637–2646
Iqbal SS, Bahadar A, Hossain N, Gull N, Ahmad TS, Ehsan N, Khan SU, Riaz T (2021) Synergetic influence of F-MWCNTS on polyvinylpyrrolidone sodium alginate composite membrane for reverse osmosis. J Environ Chem Eng 9:106085
Jadhav H, Jadhav A, Takkalkar P, Hossain N, Nizammudin S, Zahoor M, Jamal M, Mubarak NM, Griffin G, Kao N (2020) Potential of polylactide based nanocomposites-nanopolysaccharide filler for reinforcement purpose: a comprehensive review. J Polym Res 27:330
Jamshaid F, Ahmad A, Adrees M, Iqbal SS, Zaheer H, Jamil T, Ahmad J, Hussain T (2017) Tuning the interlaminar shear strength and thermo-mechanical properties of glass fiber composites by incorporation of (3-mercaptopropyl) trimethoxysilane-functionalized carbon black. Iran Polym J 26:913–927
Javaid A (2005) Membranes for solubility-based gas separation applications. Chem Eng J 112:219–226
J-M Duval, B Folkers HV Mulder (1994) Descjrandchamps,~, smolders' aca <Preparation of zeolite filled glassy polymer membranes.pdf>. polymer science 54, 409–418
Kammermeyer K (1957) Silicone rubber as a selective barrier. Ind Eng Chem Res 49:1685
Kim S, Pechar TW, Marand E (2006) Poly (imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 192:330–339
Li Z, Nambiar S, Zheng W, Yeow J (2013) PDMS/single-walled carbon nanotube composite for proton radiation shielding in space applications. Mater Lett 108:79–83
Liu CSTW, Kulprathipanja S (2010) Crosslinked organic-inorganic hybrid membranes and their use in gas separation, in. Honeywell UOP United States Patent No 20090299015:2010
Mahurin SM, Lee JS, Baker GA, Luo H, Dai S (2010) Performance of nitrile-containing anions in task-specific ionic liquids for improved CO2/N2 separation. J Membr Sci 353:177–183
Mazari SA, Ali E, Abro R, Khan FSA, Ahmed I, Ahmed M, Nizamuddin S, Siddiqui TH, Hossain N, Mubarak NM (2021) Nanomaterials: applications, waste-handling, environmental toxicities, and future challenges-a review. J Environ Chem Eng. 105028
Mubashir M, Yeong YF, Lau KK (2016) Ultrasonic-assisted secondary growth of deca-dodecasil 3 rhombohedral (DD3R) membrane and its process optimization studies in CO2/CH4 separation using response surface methodology. J Nat Gas Sci Eng 30:50–63
Mubashir M, Yeong YF, Lau KK, Chew TL, Norwahyu J (2018) Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53 (Al)/cellulose acetate (CA) mixed matrix membranes. Separ Sci Technol 199:140–151
Mubashir M, Dumée LF, Fong YY, Jusoh N, Lukose J, Chai WS, Show PL (2021) Cellulose acetate-based membranes by interfacial engineering and integration of ZIF-62 glass nanoparticles for CO2 separation. J Hazard Mater 415:125639
Mubashir M, Ashena R, Bokhari A, Mukhtar A, Saqib S, Ali A, Saidur R, Khoo KS, Ng HS, Karimi F (2022) Effect of process parameters over carbon-based ZIF-62 nano-rooted membrane for environmental pollutants separation. Chemosphere 291:133006
Nour M, Berean K, Balendhran S, Ou JZ, Du Plessis J, McSweeney C, Bhaskaran M, Sriram S, Kalantar-zadeh K (2013) CNT/PDMS composite membranes for H2 and CH4 gas separation. Int J Hydrogen Energy 38:10494–10501
Nowakowski P, Villain S, Kopia A, Suliga I, Gavarri J-R (2008) Catalytic conversion of air–methane flow by nanostructured ruthenium dioxide: FTIR spectroscopy and modeling. Appl Surf Sci 254:5675–5682
Okolo C, Rafique R, Iqbal SS, Saharudin MS, Inam F (2020) Carbon nanotube reinforced high density polyethylene materials for offshore sheathing applications. Molecules 25:2960
Pan J, Zhang L, Wang Z, Sun S-P, Cui Z, Tavajohi N (2022) Poly (vinylidene fluoride-co-hexafluoro propylene) membranes prepared via thermally induced phase separation and application in direct contact membrane distillation. Front Chem Sci Eng 16:720–730
Park HB, Jung CH, Kim YK, Nam SY, Lee SY, Lee YM (2004) Pyrolytic carbon membranes containing silica derived from poly(imide siloxane): the effect of siloxane chain length on gas transport behavior and a study on the separation of mixed gases. J Membr Sci 235:87–98
Quan S, Li SW, Xiao YC, Shao L (2017) CO2-selective mixed matrix membranes (MMMs) containing graphene oxide (GO) for enhancing sustainable CO2 capture. Int J Greenhouse Gas Control 56:22–29
Reijerkerk SR, Knoef MH, Nijmeijer K, Wessling M (2010) Poly(ethylene glycol) and poly(dimethyl siloxane): combining their advantages into efficient CO2 gas separation membranes. J Membr Sci 352:126–135
Robeson LM (1991) Correlation of separation factor versus permeability for polymeric membranes. J Membr Sci 62:165–185
Robeson LM (2008) The upper bound revisited. J Membr Sci 320:390–400
Rutnakornpituk M, Ngamdee P (2006) Surface and mechanical properties of microporous membranes of poly(ethylene glycol)–polydimethylsiloxane copolymer/chitosan. Polymer 47:7909–7917
Sagar S, Iqbal N, Maqsood A, Shahid M, Shah NA, Jamil T, Bassyouni MI (2015) Fabrication and thermal characteristics of functionalized carbon nanotubes impregnated polydimethylsiloxane nanocomposites. J Compos Mater 49:995–1006
Sanip SM, Ismail AF, Goh PS, Soga T, Tanemura M, Yasuhiko H (2011) Gas separation properties of functionalized carbon nanotubes mixed matrix membranes. Sep Purif Technol 78:208–213
Semsarzadeh MA, Ghahramani M (2015) Synthesis and morphology of polyacrylate-poly(dimethyl siloxane) block copolymers for membrane application. Macromol Res 23:898–908
Shahapurkar K, Chenrayan V, Tesfamarium BB, Soudagar MEM, Hossain N, Rajhi A, Alamri S, Alarifi IM, Shahapurkar P, Mujtaba M (2021) Parametric analysis of epoxy/crumb rubber composite by using Taguchi—GRA hybrid technique. Polymers 13:3441
Silva EAd, Windmöller D, Silva GG, Figueiredo KCdS (2017) Polydimethylsiloxane membranes containing multi-walled carbon nanotubes for gas separation. Mater Res 20:1454–1460
Viannie LR, Banapurmath N, Soudagar MEM, Nandi AV, Hosain N, Shellikeri A, Kaulgud V, Mujtaba M, Khan SA, Asif M (2021): Electrical and mechanical properties of flexible multiwalled carbon nanotube/poly (dimethylsiloxane) based nanocomposite sheets. J Environ Chem Eng 106550
Acknowledgements
The authors thank Taif University Researchers Supporting Project number (TURSP-2020/260), Taif University, Taif, Saudi Arabia.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Taif University Researchers Supporting Project number (TURSP-2020/260), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
BFF: formal analysis, methodology, writing—original draft; SSI: conceptualization, supervision; AB: supervision, formal analysis, writing—review and editing; NH: writing—original draft, proofreading; AJ: formal analysis, methodology, writing—editing final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The facts and views in the manuscript are solely ours, and we are responsible for authenticity, validity, and originality. We also declare that this manuscript is our original work and have not copied it from anywhere else. There is no plagiarism in my manuscript.
Consent for publication
We undertake and agree that the manuscript submitted to your journal has not been published elsewhere and has not been simultaneously submitted to other journals.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Felemban, B.F., Iqbal, S.S., Bahadar, A. et al. Reinforcement of single-walled carbon nanotubes on polydimethylsiloxane membranes for CO2, O2, and N2 permeability/selectivity. Environ Sci Pollut Res 30, 66800–66811 (2023). https://doi.org/10.1007/s11356-023-26962-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26962-x