Abstract
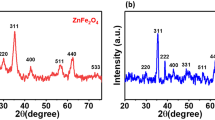
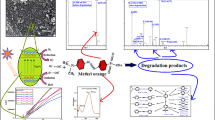
Removal of water pollutants (methylene blue dye and heavy metals) was achieved by zinc/manganese-doped nickel ferrites (Ni1 − xMxFe2O4, where x = 0.00, 0.025, 0.10). Degradation of dye was achieved under natural solar light illumination. Degradation studies of dye were conducted under different parameters such as contact time—80 min, dye’s concentration—5 mg/L, pH—7, and dosage of ferrites—15 mg. The adsorption of dye was studied using non-linear kinetics models (pseudo-first-order and pseudo-second-order) and isotherm models (Langmuir and Freundlich). The adsorption of dye followed pseudo-first-order kinetics (R2 = 0.99377) than second-order kinetics (R2 = 0.98063) and Langmuir isotherm model (R2 = 0.96095) than Freundlich model (R2 = 0.95962) with maximum adsorption efficiency of 29.62 mg/g. Doping of nickel ferrites caused an increase in the removal percentage of methylene blue dye (80 to 90%) and inorganic effluents (75 to 95% for lead and 47 to 82% for cadmium). In addition to this, band gap energy (2.43 to 3.26 eV) (UV–Vis spectroscopy), pore radius (65.2 to 74.8 A°), and specific surface area (16.45 to 27.95 m2/g) (BET analysis) were also increased. Generally, the results of the study revealed that synthesized nanoparticles can act as potential candidate for the removal of effluents from wastewater under optimum parameters along with recyclability, reusability, and separation under the influence of a magnetic field.
















Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Agency for Toxic Substance and Disease Registry (2019) ATSDR Substance Priority List. ATSDR, Atlanta, GA, USA. Available online: https://www.atsdr.cdc.gov/spl/resources/index.html. Accessed 28 Jul 2022
Ahmad A, Mohd-Setapar SH, Chuong CS, Khatoon A, Wani WA, Kumar R, Rafatullah M (2015) Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv 5:30801–30818. https://doi.org/10.1039/C4RA16959J
Ahmad U, Afzia M, Shah F, Ismail B, Rahim A, Khan RA (2022) Improved magnetic and electrical properties of transition metal doped nickel spinel ferrite nanoparticles for prospective applications. Mater Sci Semicond Process 148:106830. https://doi.org/10.1016/j.mssp.2022.106830
Amar I, Sharif A, Ali M, Alshareef S, Altohami F, Abdulqadir M, Ahwidi M (2020) Removal of methylene blue from aqueous solutions using nano-magnetic adsorbent based on zinc-doped cobalt ferrite. Chem Methodol 4:. https://doi.org/10.33945/SAMI/CHEMM.2020.1.1
Amiri M, Salavati-Niasari M, Akbari A (2019) Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv Colloid Interface Sci 265:29–44. https://doi.org/10.1016/j.cis.2019.01.003
Assadi A, Dehghani MH, Rastkari N, Nasseri S, Mahvi AH (2012) Photocatalytic reduction of hexavalent chromium in aqueous solutions with zinc oxide nanoparticles and hydrogen peroxide. Environ Prot Eng 38:5–16. https://doi.org/10.5277/EPE120401
Assar ST, Abosheiasha HF (2015) Effect of Ca substitution on some physical properties of nano-structured and bulk Ni-ferrite samples. J Magn Magn Mater 374:264–272. https://doi.org/10.1016/j.jmmm.2014.08.011
Babapour M, Dehghani MH, Alimohammadi M, Arjmand MM, Salari M, Rasuli L, Khan NA (2022) Adsorption of Cr (VI) from aqueous solution using mesoporous metal-organic framework-5 functionalized with the amino acids: characterization, optimization, linear and nonlinear kinetic models. J Mol Liq 345:117835. https://doi.org/10.1016/j.molliq.2021.117835
Balaji S, Selvan RK, Berchmans LJ, Angappan S, Subramanian K, Augustin CO (2005) Combustion synthesis and characterization of Sn4+ substituted nanocrystalline NiFe2O4. Mater Sci Eng: B 119:119–124. https://doi.org/10.1016/j.mseb.2005.01.021
Barick KC, Singh S, Aslam M, Bahadur D (2010) Porosity and photocatalytic studies of transition metal doped ZnO nanoclusters. Microporous Mesoporous Mater 134:195–202. https://doi.org/10.1016/j.micromeso.2010.05.026
Bazin I, Hassine AIH, Hamouda YH, Mnif W, Bartegi A, Lopez-Ferber M, Gonzalez C (2012) Estrogenic and anti-estrogenic activity of 23 commercial textile dyes. Ecotoxicol Environ Saf 85:131–136. https://doi.org/10.1016/j.ecoenv.2012.08.003
Bhatia N, Kumari A, Thakur N, Sharma G, Singh RR, Sharma R (2022) Phytochemically stabilized chitosan encapsulated Cu and Ag nanocomposites to remove cefuroxime axetil and pathogens from the environment. Int J Biol Macromol 248:119206. https://doi.org/10.1016/j.ijbiomac.2022.05.143
Briggs AM, Cross MJ, Hoy DG, Sanchez-Riera L, Blyth FM, Woolf AD, March L (2016) Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World Health Organization world report on ageing and health. Gerontologist 56:S243–S255. https://doi.org/10.1093/geront/gnw002
Bushkova VS, Yaremiy IP, Ostafiychuk BK, Riznychuk NI, Solovei RS (2019) Sol-gel synthesis, structure and optical properties of nickel-manganese ferrites. J NanoElectron Phys 11:03021-1–03021-5. https://doi.org/10.21272/JNEP.11(3).03021
Chatterjee S, Kumar A, Basu S, Dutta S (2012) Application of response surface methodology for methylene blue dye removal from aqueous solution using low cost adsorbent. J Chem Eng 181:289–299. https://doi.org/10.1016/j.cej.2011.11.081
da Rosa MP, Igansi AV, Lütke SF, Junior TRSAC, do Santos ACR, Inacio APDOL, Beck PH (2019) A new approach to convert rice husk waste in a quick and efficient adsorbent to remove cationic dye from water. J Environ Chem Eng 7:103504. https://doi.org/10.1016/j.jece.2019.103504
Das R, Khan GG, Varma S, Mukherjee GD, Mandal K (2013) Effect of quantum confinement on optical and magnetic properties of Pr–Cr-codoped bismuth ferrite nanowires. J Phys Chem C 117:20209–20216. https://doi.org/10.1021/jp407334d
Dasan YK, Guan BH, Zahari MH, Chuan LK (2017) Influence of La3+ substitution on structure, morphology and magnetic properties of nanocrystalline Ni-Zn ferrite. PLoS One 12:e0170075. https://doi.org/10.1371/journal.pone.0170075
Deepty M, Srinivas C, Kumar ER, Mohan NK, Prajapat CL, Rao TC, Sastry DL (2019) XRD, EDX, FTIR and ESR spectroscopic studies of co-precipitated Mn-substituted Zn–ferrite nanoparticles. Ceram Int 45:8037–8044. https://doi.org/10.1016/j.ceramint.2019.01.029
Dehghani MH, Naghizadeh A, Rashidi A, Derakhshani E (2013) Adsorption of reactive blue 29 dye from aqueous solution by multiwall carbon nanotubes. Desalin Water Treat 51:7655–7662. https://doi.org/10.1080/19443994.2013.791772
Dehghani MH, Taher MM, Bajpai AK, Heibati B, Tyagi I, Asif M, Gupta VK (2015) Removal of noxious Cr (VI) ions using single-walled carbon nanotubes and multi-walled carbon nanotubes. J Chem Eng 279:344–352. https://doi.org/10.1016/j.cej.2015.04.151
Dehghani MH, Dehghan A, Alidadi H, Dolatabadi M, Mehrabpour M, Converti A (2017a) Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: kinetic and equilibrium study. Korean J Chem Eng 34:1699–1707. https://doi.org/10.1007/s11814-017-0077-2
Dehghani MH, Dehghan A, Najafpoor A (2017b) Removing Reactive Red 120 and 196 using chitosan/zeolite composite from aqueous solutions: kinetics, isotherms, and process optimization. J Ind Eng Chem 51:185–195. https://doi.org/10.1016/j.jiec.2017.03.001
Dehghani MH, Zarei A, Mesdaghinia A, Nabizadeh R, Alimohammadi M, Afsharnia M (2017c) Adsorption of Cr (VI) ions from aqueous systems using thermally sodium organo-bentonite biopolymer composite (TSOBC): response surface methodology, isotherm, kinetic and thermodynamic studies. Desalin Water Treat 85:298–312. https://doi.org/10.5004/dwt.2017.21306
Dehghani MH, Mahdavi P, Heidarinejad Z (2018a) The experimental data of investigating the efficiency of zinc oxide nanoparticles technology under ultraviolet radiation (UV/ZnO) to remove Acid–32–Cyanine 5R from aqueous solutions. Data Brief 21:767–774. https://doi.org/10.1016/j.dib.2018.10.037
Dehghani MH, Pourshabanian M, Heidarinejad Z (2018b) Experimental data on the adsorption of Reactive Red 198 from aqueous solution using Fe3O4 nanoparticles: optimization by response surface methodology with central composite design. Data Brief 19:2126–2132. https://doi.org/10.1016/j.dib.2018.07.008
Dehghani MH, Tajik S, Panahi A, Khezri M, Zarei A, Heidarinejad Z, Yousefi M (2018c) Adsorptive removal of noxious cadmium from aqueous solutions using poly urea-formaldehyde: a novel polymer adsorbent. MethodsX 5:1148–1155. https://doi.org/10.1016/j.mex.2018.09.010
Dehghani MH, Nabizadeh R, Nazmara S, Rasoulzadeh H, Mohammadi AS, Karri RR, Sahu JN (2020) Parametric modelling of Pb (II) adsorption onto chitosan-coated Fe3O4 particles through RSM and DE hybrid evolutionary optimization framework. J Mol Liq 297:111893–111893. https://doi.org/10.1016/j.molliq.2019.111893
Dehghani MH, Salari M, Karri RR, Hamidi F, Bahadori R (2021) Process modeling of municipal solid waste compost ash for reactive red 198 dye adsorption from wastewater using data driven approaches. Sci Rep 11:1–20. https://doi.org/10.1038/s41598-021-90914-z
Dinh Du P, Hieu NT, To TC, Bach LG, Tinh MX, Xuan Mau T, Quang Khieu D (2019) Aminopropyl functionalised MCM-41 synthesis and application for adsorption of Pb (II) and Cd (II). Adv Mater Sci Eng 2019:. https://doi.org/10.1155/2019/8573451
Elgendy A, El Basiony NM, Heakal FET, Elkholy AE (2020) Mesoporous Ni-Zn-Fe layered double hydroxide as an efficient binder-free electrode active material for high-performance supercapacitors. J Power Sources 466:228294. https://doi.org/10.1016/j.jpowsour.2020.228294
Franco A Jr, e SilvaZapf FCVS (2012) High temperature magnetic properties of Co1-xMgxFe2O4 nanoparticles prepared by forced hydrolysis method. J Appl Phys 111:07B530. https://doi.org/10.1063/1.3677923
Gautam RK, Sharma SK, Mahiya S, Chattopadhyaya MC (2014) Contamination of heavy metals in aquatic media transport toxicity and technologies for remediation. Heavy Metals in Water Presence, Removal and Safety. RSC, Cambridge, pp 1–24. https://doi.org/10.1039/9781782620174-00001
Gautam S, Shandilya P, Singh VP, Raizada P, Singh P (2016) Solar photocatalytic mineralization of antibiotics using magnetically separable NiFe2O4 supported onto graphene sand composite and bentonite. J Water Process Eng 14:86–100. https://doi.org/10.1016/j.jwpe.2016.10.008
Golestanifar H, Haibati B, Amini H, Dehghani MH, Asadi A (2015) Removal of hexavalent chromium from aqueous solution by adsorption on γ-alumina nanoparticles. Environ Prot Eng 43:3067–3075. https://doi.org/10.1016/j.watres.2009.04.008
Guo D, Zhang Z, Lin M, Fan X, Chai G, Xu Y, Xue D (2009) Ni–Zn ferrite films with high resonance frequency in the gigahertz range deposited by magnetron sputtering at room temperature. J Phys D: Appl Phys 42:125006. https://doi.org/10.1088/0022-3727/42/12/125006
Gupta N, Jain P, Rana R, Shrivastava S (2017) Current development in synthesis and characterization of nickel ferrite nanoparticles. Mater Today: Proc 4:342–349. https://doi.org/10.1016/j.matpr.2017.01.031
Hamed T, John O, Emmanuel A, Benjamin K (2020) Application of green technology using biological means for the adsorption of micro-pollutants in water. J Environ Prot 11:735–752. https://doi.org/10.4236/jep.2020.119045
Hamidi F, Dehghani MH, Kasraee M, Salari M, Shiri L, Mahvi AH (2022) Acid red 18 removal from aqueous solution by nanocrystalline granular ferric hydroxide (GFH); optimization by response surface methodology & genetic-algorithm. Sci Rep 12:4761. https://doi.org/10.1038/s41598-022-08769-x
Hankare PP, Sankpal UB, Patil RP, Mulla IS, Sasikala R, Tripathi AK, Garadkar KM (2010) Synthesis and characterization of nanocrystalline zinc substituted nickel ferrites. J Alloys Compd 496:256–260. https://doi.org/10.1016/j.jallcom.2010.01.009
Hao A, He S, Qin N, Chen R, Bao D (2017) Ce-doping induced enhancement of resistive switching performance of Pt/NiFe2O4/Pt memory devices. Ceram Int 43:S481–S487. https://doi.org/10.1016/j.ceramint.2017.05.214
Hassan MM, Carr CM (2018) A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 209:201–219. https://doi.org/10.1016/j.chemosphere.2018.06.043
Hema E, Manikandan A, Karthika P, Durka M, Antony SA, Venkatraman BR (2016) Magneto-optical properties of reusable spinel NixMg1−xFe2O4 (0.0 ≤ x ≤ 1.0) nano-catalysts. J Nanosci Nanotechnol 16:7325–7336. https://doi.org/10.1166/jnn.2016.11109
Hezam FA, Nur O, Mustafa MA (2020) Synthesis, structural, optical and magnetic properties of NiFe2O4/MWCNTs/ZnO hybrid nanocomposite for solar radiation driven photocatalytic degradation and magnetic separation. Colloids Surf A Physicochem Eng Asp 592:124586. https://doi.org/10.1016/j.colsurfa.2020.124586
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manage 182:351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Huo JB, Xu L, Yang JCE, Cui HJ, Yuan B, Fu ML (2018) Magnetic responsive Fe3O4-ZIF-8 core-shell composites for efficient removal of As (III) from water. Colloids Surf A: Physicochem Eng Asp 539:59–68. https://doi.org/10.1016/j.colsurfa.2017.12.010
Iranmanesh P, Yazdi ST, Mehran M, Saeednia S (2018) Superior magnetic properties of Ni ferrite nanoparticles synthesized by capping agent-free one-step coprecipitation route at different pH values. J Magn Magn Mater 449:172–179. https://doi.org/10.1016/j.jmmm.2017.10.040
Jadhav SA, Khedkar MV, Somvanshi SB, Jadhav KM (2021) Magnetically retrievable nanoscale nickel ferrites: an active photocatalyst for toxic dye removal applications. Ceram Int 47:28623–28633. https://doi.org/10.1016/j.ceramint.2021.07.021
Jasrotia R, Singh VP, Kumar R, Verma R, Chauhan A (2019) Effect of Y3+, Sm3+ and Dy3+ ions on the microstructure, morphology, optical and magnetic properties NiCoZn magnetic nanoparticles. Results Phys 15:102544. https://doi.org/10.1016/j.rinp.2019.102544
Khan K (2014) Microwave absorption properties of radar absorbing nanosized cobalt ferrites for high frequency applications. J Supercond Nov Magn 27:453–461. https://doi.org/10.1007/s10948-013-2283-4
Khoso WA, Haleem N, Baig MA, Jamal Y (2021) Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci Rep 11:1–10. https://doi.org/10.1038/s41598-021-83363-1
Kukushkin SA, Osipov AV (2021) Spin polarization and magnetic moment in silicon carbide grown by the method of coordinated substitution of atoms. Materials 14:5579. https://doi.org/10.3390/ma14195579
Kumar PS, Ramalingam S, Sathishkumar K (2011) Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent. Korean J Chem Eng 28:149–155. https://doi.org/10.1007/s11814-010-0342-0
Kumari A, Sharma A, Sharma R, Malairaman U, Singh RR (2020) Biocompatible and fluorescent water based NIR emitting CdTe quantum dot probes for biomedical applications. Spectrochim Acta A Mol Biomol Spectrosc 248:119206. https://doi.org/10.1016/j.saa.2020.119206
Kumari C, Dubey HK, Naaz F, Lahiri P (2020b) Structural and optical properties of nanosized Co substituted Ni ferrites by coprecipitation method. Ph Transit 93:207–216. https://doi.org/10.1080/01411594.2019.1709120
Leslie-Pelecky DL, Rieke RD (1996) Magnetic properties of nanostructured materials. Chem Mater 8:1770–1783. https://doi.org/10.1021/cm960077f
Li YH, Di Z, Ding J, Wu D, Luan Z, Zhu Y (2005) Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res 39:605–609. https://doi.org/10.1016/j.watres.2004.11.004
Li B, Li D, Xia W, Zhang W (2018) Synthesis and characterization of a novel Zn-Ni and Zn-Ni/Si3N4 composite coating by pulse electrodeposition. Appl Surf Sci 458:665–677. https://doi.org/10.1016/j.apsusc.2018.07.146
Majid F, Rauf J, Ata S, Bibi I, Yameen M, Iqbal M (2019) Hydrothermal synthesis of zinc doped nickel ferrites: evaluation of structural, magnetic and dielectric properties. Z Phys Chem (N F) 233:1411–1430. https://doi.org/10.1515/zpch-2018-1305
Mehrasbi MR, Farahmandkia Z, Taghibeigloo B, Taromi A (2009) Adsorption of lead and cadmium from aqueous solution by using almond shells. Water Air Soil Pollut 199:343–351. https://doi.org/10.1007/s11270-008-9883-9
Mittal VK, Bera S, Nithya R, Srinivasan MP, Velmurugan S, Narasimhan SV (2004) Solid state synthesis of Mg–Ni ferrite and characterization by XRD and XPS. J Nucl Mater 335:302–310. https://doi.org/10.1016/j.jnucmat.2004.05.010
Moeinpour F, Kamyab S, Akhgar MR (2017) NiFe2O4 magnetic nanoparticles as an adsorbent for cadmium removal from aqueous solution. J Water Chem Technol 39:281–288. https://doi.org/10.3103/S1063455X17050058
Mohammad AM, Aliridha SM, Mubarak TH (2018) Structural and magnetic properties of Mg-Co ferrite nanoparticles. Dig J Nanomater Biostructures 13:615–623 https://www.researchgate.net/publication/326844466
Montgomery MA, Elimelech M (2007) Water and sanitation in developing countries: including health in the equation. Environ Sci Technol 41:17–24. https://doi.org/10.1021/es072435t
Muthu SS (2017) Sustainability in the textile industry. Springer, Singapore, pp 9–15. https://doi.org/10.1007/978-981-10-2639-3_2
Naik MM, Naik HS, Nagaraju G, Vinuth M, Vinu K, Rashmi SK (2018) Effect of aluminium doping on structural, optical, photocatalytic and antibacterial activity on nickel ferrite nanoparticles by sol–gel auto-combustion method. J Mater Sci Mater Electron 29:20395–20414. https://doi.org/10.1007/s10854-018-0174-y
Nishikawa E, da Silva MGC, Vieira MGA (2018) Cadmium biosorption by alginate extraction waste and process overview in life cycle assessment context. J Clean Prod 178:166–175. https://doi.org/10.1016/j.jclepro.2018.01.025
Omer NH (2019) Water quality parameters. Water Qual-Sci Assess Policy 18:1–34. https://doi.org/10.5772/intechopen.89657
Patil MR, Shrivastava VS (2016) Adsorptive removal of methylene blue from aqueous solution by polyaniline-nickel ferrite nanocomposites: a kinetic approach. Desalin Water Treat 57:5879–5887. https://doi.org/10.1080/19443994.2015.1004594
Peymani-Motlagh SM, Sobhani-Nasab A, Rostami M, Sobati H, Eghbali-Arani M, Fasihi-Ramandi M, Rahimi-Nasrabadi M (2019) Assessing the magnetic, cytotoxic and photocatalytic influence of incorporating Yb3+ or Pr3+ ions in cobalt–nickel ferrite. J Mater Sci : Mater Electron 30:6902–6909. https://doi.org/10.1007/s10854-019-01005-9
Pottker WE, Ono R, Cobos MA, Hernando A, Araujo JF, Bruno AC, La Porta FA (2018) Influence of order-disorder effects on the magnetic and optical properties of NiFe2O4 nanoparticles. Ceram Int 44:17290–17297. https://doi.org/10.1016/j.ceramint.2018.06.190
Qi K, Xing X, Zada A, Li M, Wang Q, Liu SY, Wang G (2020) Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: experimental and DFT studies. Ceram Int 46:1494–1502. https://doi.org/10.1016/j.ceramint.2019.09.116
Reshadi MAM, Bazargan A, McKay G (2020) A review of the application of adsorbents for landfill leachate treatment: focus on magnetic adsorption. Sci Total Environ 731:138863. https://doi.org/10.1016/j.scitotenv.2020.138863
Sadeek SA, Negm NA, Hefni HH, Wahab MMA (2015) Metal adsorption by agricultural biosorbents: adsorption isotherm, kinetic and biosorbents chemical structures. Int J Biol Macromol 81:400–409. https://doi.org/10.1016/j.ijbiomac.2015.08.031
Sendhilnathan S, Rajan PI, Adinaveen T (2018) Synthesis and characterization of NiFe2O4 nanoparticles for the enhancement of direct sunlight photocatalytic degradation of methyl orange. J Supercond Nov Magn 31:3315–3322. https://doi.org/10.1007/s10948-018-4601-3
Shahbaz Tehrani F, Daadmehr V, Rezakhani AT, Hosseini Akbarnejad R, Gholipour S (2012) Structural, magnetic, and optical properties of zinc-and copper-substituted nickel ferrite nanocrystals. J Supercond Nov Magn 25:2443–2455. https://doi.org/10.1007/s10948-012-1655-5
Sharma R, Singhal S (2013) Structural, magnetic and electrical properties of zinc doped nickel ferrite and their application in photo catalytic degradation of methylene blue. Physica B Condens Matter 414:83–90. https://doi.org/10.1016/j.physb.2013.01.015
Sherlala AIA, Raman AAA, Bello MM, Asghar A (2018) A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 193:1004–1017. https://doi.org/10.1016/j.chemosphere.2017.11.093
Singh H, Chauhan G, Jain AK, Sharma SK (2017) Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. J Environ Chem Eng 5:122–135. https://doi.org/10.1016/j.jece.2016.11.030
Somvanshi SB, Jadhav SA, Khedkar MV, Kharat PB, More SD, Jadhav KM (2020) Structural, thermal, spectral, optical and surface analysis of rare earth metal ion (Gd3+) doped mixed Zn–Mg nano-spinel ferrites. Ceram Int 46:13170–13179. https://doi.org/10.1016/j.ceramint.2020.02.091
Tamilarasi K, Udhaya PA, Meena M (2022) Enhancement on the electrical and optical behaviour of ZnFe2O4 nano particles via transition metal substitution. Mater Today: Proc https://doi.org/10.1016/j.matpr.2022.05.351
Teixeira RA, Lima EC, Benetti AD, Thue PS, Cunha MR, Cimirro NF, Dotto GL (2021) Preparation of hybrids of wood sawdust with 3-aminopropyl-triethoxysilane: application as an adsorbent to remove Reactive Blue 4 dye from wastewater effluents. J Taiwan Inst Chem Eng 125:141–152. https://doi.org/10.1016/j.jtice.2021.06.007
Vigneswari T, Raji P (2017) Structural and magnetic properties of calcium doped nickel ferrite nanoparticles by co-precipitation method. J Mol Struct 1127:515–521. https://doi.org/10.1016/j.molstruc.2016.07.116
Vinnik DA, Zhivulin VE, Sherstyuk DP, Starikov AY, Zezyulina PA, Gudkova SA, Trukhanov AV (2021) Electromagnetic properties of zinc–nickel ferrites in the frequency range of 0.05–10 GHz. Mater Today Chem 20:100460. https://doi.org/10.1016/j.mtchem.2021.100460
Xiang B, Ling D, Lou H, Gu H (2017) 3D hierarchical flower-like nickel ferrite/manganese dioxide toward lead (II) removal from aqueous water. J Hazard Mater 325:178–188. https://doi.org/10.1016/j.jhazmat.2016.11.011
Yao LW, Khan FSA, Mubarak NM, Karri RR, Khalid M, Walvekar R, Dehghani MH (2022) Insight into immobilization efficiency of lipase enzyme as a biocatalyst on the graphene oxide for adsorption of azo dyes from industrial wastewater effluent. J Mol Liq 354:118849. https://doi.org/10.1016/j.molliq.2022.118849
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater 80:33–42. https://doi.org/10.1016/S0304-3894(00)00278-8
Zawar S, Atiq S, Riaz S, Naseem S (2016) Correlation between particle size and magnetic characteristics of Mn-substituted ZnFe2O4 ferrites. Superlattices Microstruct 93:50–56. https://doi.org/10.1016/j.spmi.2016.02.048
Zhong Z, Li Q, Zhang Y, Zhong H, Cheng M, Zhang Y (2005) Synthesis of nanocrystalline Ni–Zn ferrite powders by refluxing method. Powder Technol 155:193–195. https://doi.org/10.1016/j.powtec.2005.05.060
Zywitzki D, Schaper R, Ciftyürek E, Wree JL, Taffa DH, Baier DM, Devi A (2021) Chemical vapor deposition of cobalt and nickel ferrite thin films: investigation of structure and pseudocapacitive properties. Adv Mater Interfaces 8:2100949. https://doi.org/10.1002/admi.202100949
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Seema Kumari, Rahul Sharma, and Nitika Thakur (review, editing, and formal analysis). The first draft of the manuscript was written by Seema Kumari and Asha Kumari (supervision). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, S., Sharma, R., Thakur, N. et al. Removal of organic and inorganic effluents from wastewater by using degradation and adsorption properties of transition metal-doped nickel ferrite. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-26567-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-26567-4