Abstract
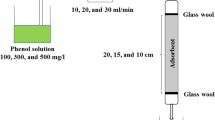
In the present study, bio-apatite/nZVI composite was synthesized through Fe(III) reduction with sodium borohydride and was fully characterized by FTIR, XRD, SEM–EDX, TEM, BET, BJH, and pHPZC. Column experiments were carried out for the removal of phosphate as a function of four operational parameters including initial phosphate concentration (100–200 mg L−1), initial solution pH (2–9), bed height (2–6 cm), and influent flow rate (2.5–7.5 mL min−1) using a response surface methodology (RSM) coupled with Box-Behnken design (BBD). 2D contour and 3D surface plots were employed to analyze the interactive effects of the four operating parameters on the column performance (e.g., uptake capacity and saturation time). According to ANOVA analysis, the influent flow rate and bed height are the most important factor on phosphate uptake capacity and saturation time, respectively. A quadratic polynomial model was excellently fitted to experimental data with a high coefficient of determination (> 0.96). The RSM-BBD model predicted maximum phosphate adsorption capacity of 85.71 mg g−1 with the desirability of 0.995 under the optimal conditions of 135.35 mg L−1, 2, 2 cm, and 7.5 mL min−1 for initial phosphate concentration, initial solution pH, bed height, and influent flow rate, respectively. The XRD analysis demonstrated that the reaction product between bio-apatite/nZVI composite and phosphate anions was Fe3 (PO4)2. 8H2O (vivianite). The suggested adsorbent can be effectively employed up to five fixed-bed adsorption–desorption cycles and was also implemented to adsorb phosphate from real samples.








Similar content being viewed by others
Data availability
My manuscript has data included as electronic supplementary material.
References
Almanassra IW, Kochkodan V, Mckay G, Kochkodan V, Atieh MA, Al-Ansari T (2020) Review of phosphate removal from water by carbonaceous sorbents. J Environ Manage 287:112245
Almanassra IW, Mckay G, Kochkodan V, Atieh MA, Al-Ansari T (2021) A state of the art review on phosphate removal from water by biochars. Chem Eng J 409:128211
Almeelbi T, Bezbaruah A (2012) Aqueous phosphate removal using nanoscale zero-valent iron. J Nanoparticle Res 14:197–210
Amiri MJ, Abedi-Koupai J, Eslamian SS, Arshadi M (2016) Adsorption of Pb(II) and Hg(II) ions from aqueous single metal solutions by using surfactant-modified ostrich bone waste. Desalin Water Treat 57:16522–16539
Amiri MJ, Abedi-Koupai J, Eslamian SS, Mousavi SF, Hasheminejad H (2013) Modeling Pb(II) adsorption from aqueous solution by ostrich bone ash using adaptive neural-based fuzzy inference system. J Environ Sci Health Part A 48(5):543–558
Amiri MJ, Abedi-koupai J, Eslamian S (2017) Adsorption of Hg(II) and Pb(II) ions by nanoscale zero-valent iron supported on ostrich bone ash in a fixed-bed column system. Water Sci Technol 76(3):671–682
Amiri MJ, Bahrami M, Beigzadeh B, Gil A (2018a) A response surface methodology for optimization of 2,4-dichlorophenoxyacetic acid removal from synthetic and drainage water: a comparative study. Environ Sci Pollut Res 25:34277–34293
Amiri MJ, Bahrami M, Dehkhodaie F (2019) Optimization of Hg(II) adsorption on bio-apatite based materials using CCD-RSM design: characterization and mechanism studies. J Water Health. 17:556–567
Amiri MJ, Mahmoudi MR, Khozaei M (2020a) Fixed bed column modeling of Cd(II) dsorption on bone char using backward Bayesian multiple linear regression. Pollution 6:451–461
Amiri MJ, Roohi R, Gil A (2018b) Numerical simulation of Cd(II) removal by ostrich bone ash supported nanoscale zero-valent iron in a fixed-bed column system: utilization of unsteady advection-dispersion-adsorption equation. J Water Process Eng 25:1–14
Amiri MJ, Roohi R, Arshadi M, Abbaspourrad A (2020b) 2,4-D adsorption from agricultural subsurface drainage by canola stalk-derived activated carbon: insight into the adsorption kinetics models under batch and column conditions. Environ Sci Pollut Res 27:16983–16997
Rice EW, Baird RB, Eaton AD (2017) Standard methods for the examination of water and wastewater, 23st edn. American Public Health Association, Washington, DC, USA
Arshadi M, EtemadGholtash J, Zandi H, Foroughifard S (2015a) Phosphate removal by a nano-biosorbent from the synthetic and real (Persian Gulf) water samples. RSC Adv 5:43290–43302
Arshadi M, Foroughifard S, EtemadGholtash J, Abbaspourrad A (2015b) Preparation of iron nanoparticles-loaded Spondias purpurea seed waste as an excellent adsorbent for removal of phosphate from synthetic and natural waters. J Colloid Interface Sci 452:69–77
Arshadi M, Abdolmaleki MK, Eskandarloo H, Azizi M, Abbaspourrad A (2018) Synthesis of highly monodispersed, stable and spherical NZVI of 20–30 nm on filter paper for the removal of phosphate from wastewater: batch and column study. ACS Sustain Chem Eng 6:11662–11676
Bahrami M, Amiri MJ, Bagheri F (2019) Optimization of the lead removal from aqueous solution using two starch based adsorbents: design of experiments using response surface methodology (RSM). J Environ Chem Eng 7:102793
Bahrami M, Amiri MJ, Beigzadeh B (2018) Adsorption of 2, 4-dichlorophenoxyacetic acid using rice husk biochar, granular activated carbon, and multi-walled carbon nanotubes in a fixed bed column system. Water Sci Technol 78:1812–1821
Cavas L, Karabay Z, Alyuruk H, Dogan H, Demir GK (2011) Thomas and artificial neural network models for the fixed-bed adsorption of methylene blue by a beach waste Posidonia oceanica (L.) dead leaves. Chem Eng J 171(2):557–562
Chowdhury S, Saha P (2013) Artificial neural network (ANN) modeling of adsorption of methylene blue by NaOH-modified rice husk in a fixed-bed column system. Environ Sci Pollut Res 20(2):1050–1058
Crane RA, Scott T (2014) The removal of uranium onto carbon-supported nanoscale zero-valent iron particles. J Nanopart Res 16:2813
Cruz-Olivares J, Pérez-Alonso C, Barrera-Díaz C, Ureña-Nuñez F, Chaparro-Mercado MC, Bilyeu B (2013) Modeling of lead (II) biosorption by residue of allspice in a fixed-bed column. Chem Eng J 228:21–27
Eslamian SS, Amiri MJ, Abedi-Koupai J, Shaeri-Karimi S (2013) Reclamation of unconventional water using nano zero-valent iron particles: an application for groundwater. Int J Water 7(1/2):1–13
Gil A, Amiri MJ, Abedi-Koupai J, Eslamian S (2018) Adsorption/reduction of Hg (II) and Pb (II) from aqueous solutions by using bone ash/nZVI composite: effects of aging time, Fe loading quantity and co-existing ions. Environ Sci Pollut Res 25:2814–2829
Gu B-W, Lee C-G, Park S-J (2018) Application of response surface methodology and semi-mechanistic model to optimize fluoride removal using crushed concrete in a fixed-bed column. Environ Technol 39:616–627
Hu A, Ren G, Che J, Guo Y, Ye J, Zhou S (2020) Phosphate recovery with granular acid-activated neutralized red mud: fixed-bed column performance and breakthrough curve modelling. J Environ Sci 90:78–86
Huang Y, Lee X, Macazo FC (2018) A sustainable adsorbent for phosphate removal: modifying multi-walled carbon nanotubes with chitosan. J Mater Sci 53:12641–12649
Li T, Liao T, Su X, Yu X, Han B, Zhu Y, Zhang Y (2018) Preparation of cobalt-containing spinel oxides as novel adsorbents for efficient phosphate removal. Environ Sci Water Res Technol 4:1671–1684
Liu Y, Liu F, Ni L, Meng M, Meng X, Zhong G, Qiu J (2016) A modeling study by response surface methodology (RSM) on Sr(II) ion dynamic adsorption optimization using a novel magnetic ion imprinted polymer. RSC Adv 6:54679–54692
Luo X, Wang X, Bao S, Liu X, Zhang W, Fang T (2016) Adsorption of phosphate in water using one-step synthesised zirconium-loaded reduced graphene oxide. Sci Rep 6:1–13
Ma F, Zhao B, Diao J, Jiang Y, Zhang J (2020) Mechanism of phosphate removal from aqueous solutions by biochar supported nanoscale zero-valent iron. RSC Adv 10:39217–39225
Maamoun I, Eljamal O, Khalil AME, Sugihara Y, Matsunaga N (2018) Phosphate removal through nano-zero-valent iron permeable reactive barrier; column experiment and reactive solute transport modeling. Transp Porous Media 125:395–412
Malakootian M, Daneshkhah M, Hossaini H (2018) Removal of phosphates from aqueous solution by sepiolite-nano zero valent iron composite optimization with response surface methodology. Int J Environ Sci Technol 15:2129–2140
Maleki S, Karimi-Jashni A (2020) Optimization of Ni(II) adsorption onto Cloisite Na+ clay using response surface methodology. Chemosphere 246:125710
Mohammad A-T, Abdulhameed AS, Jawad AH (2019) Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: methyl orange adsorption and mechanism studies. Int J Biol Macromol 129:98–109
Nodeh HR, Sereshti H, Afsharian EZ, Nouri N (2017) Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite. J Environ Manag 197:265–274
Roy S, Das P, Sengupta S (2017) Treatability study using novel activated carbon prepared from rice husk: column study, optimization using response surface methodology and mathematical modeling. Process Saf Environ Prot 105:184–193
Srivastava V, Sharma YC, Sillanpää M (2015) Application of response surface methodology for optimization of Co(II) removal from synthetic wastewater by adsorption on NiO nanoparticles. J Mol Liq 211:613–620
Singh AK, Singh KP (2018) Optimization of phosphate removal from aqueous solution using activated carbon supported zero-valent iron nanoparticles: application of RSM approach. Appl Water Sci 8:226
Wantala K, Khongkasem E, Khlongkarnpanich N, Sthiannopkao S, Kim K-W (2012) ptimization of As(V) adsorption on Fe-RH-MCM-41-immobilized GAC using Box-Behnken design: effects of pH, loadings, and initial concentrations. Appl Geochemistry 27:1027–1034
Wen Z, Zhang Y, Dai C (2014) Removal of phosphate from aqueous solution using nanoscale zerovalent iron (nZVI). Colloids Surf A Physicochem Eng Asp 457:433–440
Wu D, Shen Y, Ding A, Qiu M, Yang Q, Zheng S (2013) Phosphate removal from aqueous solutions by nanoscale zero-valent iron. Environ Technol 34:2663–2669
Yun JH (2000) Unusual adsorber dynamics due to s-shaped equilibrium isotherm. Korean J Chem Eng 17:613–617
Zhang X, Lin S, Chen Z, Megharaj M, Naidu R (2011) Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: reactivity, characterization and mechanism. Water Res 45:3481–3488
Zeng H, Lu L, Gong Z, Guo Y, Mo J, Zhang W, Li H (2019) Nanoscale composites of hydroxyapatite coated with zero valent iron: preparation, characterization and uranium removal. J Radioanal Nucl Chem 320:165–177
Acknowledgements
The authors would like to thank Fasa University for supporting of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We verify that we have seen and have approved the submitted manuscript. Our manuscript does not report on or involve the use of any animal or human data or tissue.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amiri, M.J. Synthesis and optimization of spherical nZVI (20–60 nm) immobilized in bio-apatite-based material for efficient removal of phosphate: Box-Behnken design in a fixed-bed column. Environ Sci Pollut Res 29, 67751–67764 (2022). https://doi.org/10.1007/s11356-022-20565-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20565-8