Abstract
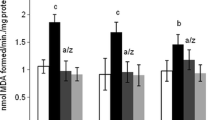
Premna odorata Blanco (Lamiaceae) is an ethnomedicinal plant, where some reports claimed their anti-inflammatory, cytotoxic, and antituberculosis effects, without investigating its role on the brain. Therefore, forty mature male rats were equally divided into 4 groups; the 1st was kept as control. Rats in groups 2 and 4 were orally given P. odorata extract daily at a dose of 500 mg/kg B.W., while those in groups 3 and 4 were daily administrated aluminum chloride “AlCl3” (70 mg/kg B.W.). The treatments extended for 30 successive days. At the end of the experimental period, brain samples were collected for biochemical assay of glutathione reductase (GSH), catalase, malondialdehyde (MDA), and acetylcholinesterase activity (AChE). Besides, monoamines (norepinephrine, dopamine, serotonin), amino acids (glutamine, serine, arginine, taurine and gamma-aminobutyric acid (GABA)), neurotransmitters, DNA damage, cyclooxygenase-2 (COX-2), and tumor necrosis factor (TNF)-α genes were estimated. Moreover, brain samples were obtained for histopathological investigation. Aluminum toxicity resulted in a decline of GSH concentration, elevation of MDA, and AChE activity. Except for GABA which exhibited a significant decrease, there was a marked increase in the measured amino acid and monoamine neurotransmitters. Also, an increase in mRNA expressions of TNF-α and COX-2 was detected. It was noticed that Premna odorata extract reduced the oxidative stress and counteracted the augmentations in AChE caused by AlCl3. Marked improvements in most measured neurotransmitters with downregulation of pro-inflammatory gene expression were recorded in P. odorata + AlCl3 group. Premna odorata restores the altered histopathological feature induced by AlCl3. In conclusion, the present findings clarify that P. odorata extract could be important in improving and treatment of neurodegenerative disorders as it was able to reduce oxidative stress, DNA damage, biochemical alterations, and histopathological changes in rats exposed to AlCl3 toxicity.



Similar content being viewed by others
References
Abdelghany AK, Khalil F, Azeem NMA, El-Nahass ES, El-Kashlan AM, Emeash HH (2019) Ginseng and moringa olifera ameliorated cognitive impairments induced by aluminium chloride in albino rat. Adv Anim Vet Sci 7(7):557–565
Abu-Taweel GM, Ajarem JS, Ahmad M (2012) Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav 101:49–56
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alzahrani YM, Sattar MA, Kamel FO, Ramadan WS, Alzahrani YA (2020) Possible combined effect of perindopril and Azilsartan in an experimental model of dementia in rats. Saudi Pharm J 28:574–581. https://doi.org/10.1016/j.jsps.2020.03.00
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol 10:399
Azib L, Debbache-Benaida N, Da Costa G, Atmani-Kilani D, Saidenea N, Ayouni K, Richard T, Atmani D (2019) Pistacialentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind Crop Prod 137:576–584
Banchroft JD, Stevens A, Turner D (1996) Theory and practice of histologic techniques, 4th edn. Churchil Livingstone, New York, London, San Francisco, Tokyo
Bhalla P, Garg ML, Dhawan DK (2010) Protective role of lithium during aluminium-induced neurotoxicity. Neurochem Int 56:256–262
Ciarlone AE (1978) Further modification of a fluorometric method for analyzing brain amines. Microchem J 23(1):9–12
Cui T, Qiu H-M, Huang D, Zhou Q, Fu XY, Li HY, Jiang XH (2017) Abnormal levels of seven amino neurotransmitters in depressed rat brain and determination by HPLC - FLD. Biomed Chromatogr 31:e3937
Dalla Torre G, Mujika JI, Lachowicz JI, Ramos MJ, Lopez X (2019) The interaction of aluminum with catecholamine-based neurotransmitters: can the formation of these species be considered a potential risk factor for neurodegenerative diseases? Dalton Trans 48(18):6003–6018. https://doi.org/10.1039/c8dt04216k
Devi KP, Sreepriya M, Devaki T, Balakrishna K (2003) Antinociceptive and hypnotic effects of Premna tomentosa L. (Verbenaceae) in experimental animals. Pharmacology. Biochem Behav 75:261–264
Dhivya Bharathi M, Justin-Thenmozhi A, Manivasagam T, Ahmad Rather M, Saravana Babu C, Mohamed Essa M, Guillemin GJ (2019) Amelioration of aluminum maltolate-induced inflammation and endoplasmic reticulum stress-mediated apoptosis by tannoid principles of Emblica officinalis in neuronal cellular model. Neurotox Res 35(2):318–330
Ellman GL, Courtney KD, Andres VJ, Feather-stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Elmaidomy AH, Mohyeldin MM, Ibrahim MM, Hassan HM, Amin E, Rateb ME, Hetta MH, El Sayed KA (2017) Acylated iridoids and rhamnopyranoses from Premna odorata (Lamiaceae) as novel mesenchymal–epithelial transition factor receptor inhibitors for the control of breast cancer. Phytother Res 31:1546–1556
Elmaidomy AH, Mohammed R, Owis AI, Hetta MH, AboulMagd AM, Siddique A, Abdelmohsen UR, Rateb ME, El Sayed KA, Hassan HM (2020) Triple-negative breast cancer suppressive activities, antioxidants and pharmacophore model of new acylated rhamnopyranoses from Premna odorata. RSC Adv 10:10584–10598
El-Rahman SSA (2003) Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol Res 47:189–194
Ghribi O, DeWitt DA, Forbes MS, Herman MM, Savory J (2001) Co-involvement of mitochondria and endoplasmic reticulum in regulation of apoptosis: changes in cytochrome c, Bcl-2 and Bax in the hippocampus of aluminum-treated rabbits. Brain Res 903:66–73. https://doi.org/10.1016/S0006-8993(01)02406-4
Gonçalves PP, Silva VS (2007) Does neurotransmission impairment accompany aluminium neurotoxicity? J Inorg Biochem 101:1291–1338
Gorun V, Proinov I, Baltescu V, Balaban G, Barzu O (1978) Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal Biochem 86(1):324–326
Hang NT, Ky PT, Minh CV, Cuong NX, Thao NP, Kiem PV (2008) Study on the chemical constituents of Premna integrifolia L.Natural. Product Commun 3(9):1449–1452
Hassan HA, Serage HM, Gad W (2015) Black berry juice attenuates neurological disorders and oxidative stress associated with concurrent exposure of aluminum and fluoride in male rats. Egypt J Basic Appl Sci 2:281–288
HelmyAbdou KA, Ahmed RR, Ibrahim MA, Abdel-Gawad DRI (2019) The anti-inflammatory influence of Cinnamomum burmannii against multi-walled carbon nanotube-induced liver injury in rats. Environ Sci Pollut Res Int 26(35):36063–36072
Hien TT, Mai NT, Tam BT, Hien NT, Cuong CVL, Anh HLT (2019) Glycosides isolated from the aerial parts of premna integrifolia growing in Thai Binh. Vietnam J Sci Technol 57(4):420–427. https://doi.org/10.15625/2525-2518/57/3/13076
Hussien HM, Abd-Elmegied A, Ghareeb DA, Hafez HS, Ahmed HEA, El-Moneam NA (2018) Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem Toxicol 111:432–444. https://doi.org/10.1016/j.fct.2017.11.025
Ibraheem EM, Mohamed EW, Taher MA (2011) Perturbation of brain neurotransmitters by aluminum in male rats and the potential role of sage. Egypt J Exp Biol (Zool) 7(2):249–259
Ibrahim MA, Ibrahem MD (2020) Acrylamide-induced hematotoxicity, oxidative stress, and DNA damage in liver, kidney, and brain of catfish (Clarias gariepinus). Environ Toxicol 35(2):300–308
Ibrahim MA, Radwan MI, Kim HK, Han J, Warda M (2020) Evaluation of global expression of selected genes as potential candidates for internal normalizing control during transcriptome analysis in dromedary camel (camelus dromedarius). Small Rumin Res 184:106050
Justin-Thenmozhi A, Dhivya Bharathi M, Kiruthika R, Manivasagam T, Borah A, Essa MM (2018) Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT/GSK-3β pathway by Hesperidin in Wistar rats. Neurotox Res 34(3):463–476
Kabra A, Kabra R, Baghel US (2015) Premna Species: A Review. J Biol Chem Chron 1(1):55–59
Kadhem WM, Enaya H (2018) Effect of lead and aluminium in the levels of dopamine and acetylcholine in the brain male rats. Res J Pharm Tech 11(5):1–3
Kaizer RR, Corrêa MC, Gris LR, da Rosa CS, Bohrer D, Morsch VM, Maria Rosa CS (2008) Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res 33:2294–2301
Kandeil MA, Mohammed ET, Hashem KS, Aleya L, Abdel-Daim MM (2019) Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ Sci Pollut Res:1–16. https://doi.org/10.1007/s11356-019-05514-2
Khalaf AA, Ibrahim MA, Tohamy AF, Abd Allah AA, Zaki AR (2017) Protective effect of vitazinc on chlorsan induced oxidative stress, genotoxicity and histopathological changes in testicular tissues of male rats. Int J Pharmacol 13:22–32
Khalaf AA, Ahmed WM, Moselhy WA, Abdel-Halim BR, Ibrahim MA (2019) Protective effects of selenium and nano-selenium on bisphenol-induced reproductive toxicity in male rats. Hum Exp Toxicol 38(4):398–408
Khatun H, Majumder R, Mamun A, Alam EK, Jami SI, Alam B (2014) Preliminary pharmacological activity of the methanolic extract of Premna integrifolia barks in rats. Avicenna J Phytomed 4(3):215–224
Kinawy AA (2019) The potential roles of aluminum chloride and sodium fluoride on the neurotoxicity of the cerebral cortex, hippocampus, and hypothalamus of male rat offspring. J Basic Appl Zool 80:17
Kumar S (1999) Aluminium-induced biphasic effect. Med Hypotheses 52(6):557–559
Kumar S (2002) Aluminium-induced changes in the rat brain serotonin system. Food Chem Toxicol 40:1875–1880
Kumar A, Dogra S, Prakash A (2009) Protective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioural and biochemical alterations in rats. Behav Brain Res 205(2):384–390
Laabbar W, Elgota A, Kissani N, Gamrani H (2014) Chronic aluminum intoxication in rat induced both serotonin changes in the dorsal raphe nucleus and alteration of glycoprotein secretion in the subcommissural organ: Immunohistochemical study. Neurosci Lett 577:72–76
Lakshmi BV, Sudhakar M, Aniska M (2014) Neuroprotective role of hydroalcoholic extract of Vitis vinifera against aluminium-induced oxidative stress in rat brain. Neurotoxicology 41:73–79
Liang J-Q, Wang L, He J-C, Hua X-D (2016) Verbascoside promotes the regeneration of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra. Neural Regen Res 11:101
Liaquat L, Sadir S, Batool Z, Tabassuma S, Shahzada S, Afzal A, Haider S (2019) Acute aluminum chloride toxicity revisited: study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci 217:202–211
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Martínez-Cué C, Rueda N (2020) Cellular Senescence in Neurodegenerative Diseases. Front Cell Neurosci 14:16. https://doi.org/10.3389/fncel.2020.00016 eCollection 2020
Marzouk MM, Ibrahim LF, El-Hagrassi AM, Fayed DB, Elkhateeb A, Abdel-Hameed ES, Hussein SR (2020) Phenolic profiling and anti-Alzheimer’s evaluation of Eremobiumaegyptiacum. Orient Pharm Exp Med 20:233–241. https://doi.org/10.1007/s13596-019-00408-7
Matyia E (2000) Aluminum enhances glutamate-mediated neurotoxicity in organotypic cultures of rat hippocampus. Folia Neuropathol 38(2):47–53
Mohamed NE, Abd El-Moneim AE (2017) Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition 35:93–99
Musalmah M, Leow KS, Nursiati MT, Raja Najmi Hanis RI, Fadly Syah A, Renuka S, Siti Norsyamimi MS, Mohamad Fairuz Y, Azian AL (2013) Selective uptake of alpha-tocotrienol and improvement in oxidative status in rat brains following short- and long term intake of tocotrienol rich fraction. Malays J Nutr 19(2):251–259
Nehru B, Anand P (2005) Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol 19:203–208
Ouyang QQ, Zhao S, Li SD, Song C (2017) Application of chitosan, chitooligosaccharide, and their derivatives in the treatment of Alzheimer’s disease. Marine Drugs 15:322–337. https://doi.org/10.3390/md15110322
Peng XM, Gao L, Huo SX, Liu XM, Yan M (2015) The mechanism of memory enhancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of d-gal and AlCl3. Phytother Res 29:1137–1144
Pereira M, Dombrowski PA, Losso EM, Chioca LR, Da Cunha C, Andreatini R (2011) Memory impairment induced by sodium fluoride is associated with changes in brain monoamine levels. Neurotox Res 19(1):55–62
Rather MA, Thenmozhi AJ, Manivasagam T, Bharathi DM, Essa MM and Guillemin GJ (2018) Neuroprotective role of Asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Front Biosci, Scholar, 10: 262-275
Said MM, Abd Rabo MM (2017) Neuroprotective effects of eugenol against aluminium-induced toxicity in the rat brain. Arh Hig Rada Toksikol 68:27–37
Sánchez-Iglesias S, Méndez-Álvarez E, Iglesias-González J, Muñoz-Patiño A, Sánchez-Sellero I, Labandeira-García JL, Soto-Otero R (2009) Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J Neurochem 109:879–888. https://doi.org/10.1111/j.1471-4159.2009.06019.x
Schad AH, Fahimi D, Völkl A, Baumgart E (2003) Expression of catalase mRNA and protein in adult rat brain: detection by nonradioactive in situ hybridization with signal amplification by catalyzed reporter deposition (ISH–CARD) and immunohistochemistry (IHC)/immunofluorescence (IF). J Histochem Cytochem 51(6):751–760
Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M, Sabban EL (1999) Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry 45:853–862
Singh T, Goel RK (2015) Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. NeuroToxicology 49:1–7
Singla N, Dhawan DK (2017) Zinc improves cognitive and neuronal dysfunction during aluminium-induced neurodegeneration. Mol Neurobiol 54:406–422
Strużyńska L, Sulkowski G (2004) Relationships between glutamine, glutamate, and GABA in nerve endings under Pb-toxicity conditions. J Inorg Biochem 98:951–958
Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68(10):930–941
Taïr K, Kharoubi O, Taïr OA, Hellal N, Benyettou I, Aoues A (2016) Aluminium-induced acute neurotoxicity in rats: treatment with aqueous extract of Arthrophytum (Hammadascoparia). J Acute Dis 5(6):470–482
Tian F, Yu L, Zhai Q, Xiao Y, Shi Y, Jiang J, Liu X, Zhao J, Zhang H, Chen W (2017) The therapeutic protection of a living and dead Lactobacillus strain against aluminum-induced brain and liver injuries in C57BL/6mice. PLoS One 12(4):e0175398. https://doi.org/10.1371/journal.pone.0175398
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicine 5:93
Uchiyama M, Mihara M (1978) Determination of malonaldehydeprecursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Van Doorn R, Leijdekkers C-M, Henderson PT (1978) Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicology 11:225–233
Wei Y, Liu D, Zheng Y, Li H, Hao C, Ouyang W (2017) Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res Bull 134:262–272
Wei W, Lu M, Lan X, Liu N, Wang H, Du J, Sun T, Li Y, Yu J (2019) Neuroprotective effect of verbascoside on hypoxic-ischemic brain damage in neonatal rat. Neurosci Lett 711:134415
Wu LL, Yan L, Yan C, Yi P, Su JF, Wu WK (2016) Antidepressant-like effects of fractions prepared from Danzhi-xiaoyao-san decoction in rats with chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal axis, arginine vasopressin, and neurotransmitters. Evid Based Complement Alternat Med 3:1–11
Yokel RA, Allen DD, Ackley DC (1999) The distribution of aluminum into and out of the brain. J InorgBiochem 76(2):127–132
Zaky A, Bassiouny A, Farghaly M, El-Sabaa BM (2017) A combination of resveratrol and curcumin is effective against aluminum chloride-induced Neuroinflammation in Rats. J Alzheimers Dis 60(s1):S221–S235
Zhao Y, Wang D, Bais S, Wang H (2019) Modulation of pro-inflammatory mediators by eugenol in AlCl3 induced dementia in rats. Int J Pharmacol 15:457–464
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 195 kb)
Rights and permissions
About this article
Cite this article
Ahmed, W.M.S., Helmy, N.A., Ibrahim, M.A. et al. Premna odorata extract as a protective agent on neurotoxic effect of aluminum: neurochemical, molecular, and histopathological alterations. Environ Sci Pollut Res 28, 2146–2157 (2021). https://doi.org/10.1007/s11356-020-10659-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10659-6