Abstract
The dyes Auramine and Auramine O are used in several industrial products, despite the scarce information regarding their ecotoxicity. The aim of the present study was to assess the acute and chronic toxicity of both dyes to aquatic organisms from different trophic levels (Raphidocelis subcapitata, Daphnia similis, Hydra attenuata, and Danio rerio) and calculate their predicted non-effect concentrations (PNEC). Auramine and Auramine O induced toxicity to all selected test organisms with L(E)C50 values ranging from 300 to 4800 ug/L. Both dyes induced inhibition in the growth rate of exposed algae, negatively affecting the reproduction of D. similis and induced deformities in H. attenuata (clubbed tentacles and shortened tentacles) and D. rerio (edemas, tail malformation and delay in yolk sac absorption). PNEC values of 0.92 μg/L and 4.0 μg/L were obtained for Auramine and Auramine O, respectively, based on results of the most sensitive test system (algae). Test results were analyzed using the Criteria of Reporting and Evaluating Ecotoxicity Data (CRED), confirming their reliability and relevance. Thus, PNEC values can be used in future risk assessments of those substances in freshwater systems.




Similar content being viewed by others
References
Abe FR, Mendonça JN, Moraes LAB, Oliveira GAR, Gravato C, Soares AMVM, Oliveira DP (2017) Toxicological and behavioral responses as a tool to assess the effects of natural and synthetic dyes on zebrafish early life. Chemosphere 178:282–290. https://doi.org/10.1016/j.chemosphere.2017.03.030
ABNT (2016) Aquatic Ecotoxicology - Acute Toxicity - Bioassay Methodology with Daphnia Spp (Crustacea, Cladocera). NBR 12713. Brazilian Association of Technique Standards
de Aragão Umbuzeiro G, Freeman HS, Warren SH et al (2005) The contribution of azo dyes to the mutagenic activity of the Cristais River. Chemosphere 60:55–64. https://doi.org/10.1016/j.chemosphere.2004.11.100
Azevedo CCJ, Kasahara MK, Montagner CC (2020) Development and validation of method to quantify auramine dyes in toxicity tests and samples environmental using HPLC/DAD. Submitted as Quim Nova.
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigments 73:81–85
Bafana A, Devi S, Chakrabarti T (2011) Azo dyes: past, present and the future. Environ Rev 370:350–370. https://doi.org/10.1139/A11-018
Carneiro PA, Oliveira DP, Umbuzeiro GA, Zanoni MVB (2010) Mutagenic activity removal of selected disperse dye by photoeletrocatalytic treatment. J Appl Electrochem 40:485–492. https://doi.org/10.1007/s10800-009-0018-9
Christian Ritz JCS (2005) Bioassay Analysis Using R
Croce R, Cinà F, Lombardo A, Crispeyn G, Cappelli CI, Vian M, Maiorana S, Benfenati E, Baderna D (2017) Aquatic toxicity of several textile assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol Environ Saf 144:79–87
Dach K, Yaghoobi B, Schmuck MR et al (2019) Teratological and behavioral screening of the National Toxicology Program 91-Compound Library in Zebrafish ( Danio rerio ). 167:77–91. https://doi.org/10.1093/toxsci/kfy266
Environment Canada (2016) Environment Canada Certain solvent dyes of the Aromatic Azo and Benzidine-based substance grouping - Canada. https://www.canada.ca/en/healthcanada/services/chemical-substances/fact-sheets/chemicals-glance/certain-solvent-dyes-aromatic-benzidine-based-substance-grouping.html. Accessed 13 Jun 2019
European Commission (2011) Common implementation strategy for the Water Framework Directive (2000/60/EC). Guidance Document No. 27. Technical guidance for deriving Environmental Quality Standards. European Union. 204 pp.
Ferraz ERA, Umbuzeiro GA, de Almeida G et al (2011) Differential toxicity of Disperse Red 1 and Disperse Red 13 in the Ames test, HepG2 cytotoxicity assay, and Daphnia acute toxicity test. Environ Toxicol 26:489–497. https://doi.org/10.1002/tox.20576
Gessner T, Mayer U (2000) Triarylmethane and Diarylmethane Dyes. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, In
Ghate HV, Dodakundi GB (1978) Effect of dye factory effluent on the developing embryos of microphyla O. Indian J Environ Health 20:359–365
GHS (2009) Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Globally Harmonized System, GHS, New York and Geneva, United Nations. ST/SG/AC.10/30/Rev.3.
Guaratini CCI, Zanoni MVB (2000) Corantes têxteis. Quim Nova 23:71–78
Hernández-Zamora M, Martínez-Jerónimo F (2019) Congo red dye diversely affects organisms of different trophic levels: a comparative study with microalgae, cladocerans, and zebrafish embryos. Environ Sci Pollut Res 26:11743–11755. https://doi.org/10.1007/s11356-019-04589-1
IARC (2010) IARC Monographs on the evaluation of carcinogenic risks to humans VOLUME 99 Some Aromatic Amines, Organic Dyes, and Related Exposures
Jiang L-L, Li K, Yan D-L, Yang M-F, Ma L, Xie L-Z (2020) Toxicity assessment of 4 azo dyes in zebrafish embryos. Int J Toxicol 39(2):115–123
de Jong L, Pech N, de Aragão Umbuzeiro G, Moreau X (2016) Multi-scale biomarker evaluation of the toxicity of a commercial azo dye (Disperse Red 1) in an animal model, the freshwater cnidarian Hydra attenuata. Water Res 96:62–73. https://doi.org/10.1016/j.watres.2016.03.043
Joshi V, Pancharatna K (2018) Food colorant Sunset Yellow (E110) intervenes developmental profile of zebrafish. J Appl Toxicol 39(4):571–581
Jung H, Seok S-H, Han J-H, Abdelkader TS, Kim T-H, Chang S-N, Ko A-S, Choi S-K, Lee C-R, Seo J-E, Byun S-H, Kim J-A, Park J-H (2012) Effect of fluorescent whitening agent on the transcription of cell damage-related genes in zebrafish embryos. J Appl Toxicol 32(9):654–661
Jungtanasombut W, Preeprem P (2014) Effects of reactive red 239 on developing zebra fish (Danio rerio) embryos. Kasetsart J (Nat Sci) 48:619–628
Klassen CD, Casarett LJ, Doull J (2013) Casarett and doull’s toxicology: the basic science of poisons. New York.
Leite S, Souza B De, Arag G De, Pupo RF (2016) Chemosphere monitoring ecotoxicity of disperse red 1 dye during photo-Fenton degradation. 148:. https://doi.org/10.1016/j.chemosphere.2016.01.053
Li J, Ding X-M, Liu D-D, Guo F, Chen Y, Zhang YB, Liu HM (2013) Simultaneous determination of eight illegal dyes in chili products by liquid chromatography–tandem mass spectrometry. J Chromatogr B 942–943:46–52. https://doi.org/10.1016/J.JCHROMB.2013.10.010
Lin Q (2007) Simultaneous determination of chrysoidine and auramine O in bean products by HPLC. Se pu = Chinese J Chromatogr 25:776–777
Liu H, Yu H, Giesy JP, Sun Y, Wang X (2007) Toxicity of HC Orange No. 1 to Daphnia magna, Zebrafish (Brachydanio rerio) embryos, and goldfish (Carassius auratus). Chemosphere 66(11):2159–2165
Luna LAV, da Silva THG, Nogueira RFP, Kummrow F, Umbuzeiro GA (2014) Aquatic toxicity of dyes before and after photo-Fenton treatment. J Hazard Mater 276:332–338. https://doi.org/10.1016/j.jhazmat.2014.05.047
Maguire RJ (1992) Occurrence and persistence of dyes in a canadian river. 25:265–270
Manimaran D, Sulthana A s, Elangovan N (2018) Reactive black 5 induced developmental defects via potentiating apoptotic cell death in Zebrafish (Danio rerio) embryos. Pharm Pharmacol Int J 6(6)
Martelli A, Brambilla Campart G, Canonero R, Carrozzino R, Mattioli F, Robbiano L, Cavanna M (1998) Evaluation of auramine genotoxicity in primary rat and human hepatocytes and in the intact rat. Mutat Res Toxicol Environ Mutagen 414:37–47. https://doi.org/10.1016/S1383-5718(98)00037-0
Martínez-jerónimo MHF (2019) Exposure to the azo dye Direct blue 15 produces toxic effects on microalgae, cladocerans, and zebra fish embryos. Ecotoxicology 28:890–902. https://doi.org/10.1007/s10646-019-02087-1
Moermond CTA, Kase R, Korkaric M, Ågerstrand M (2016) CRED: criteria for reporting and evaluating ecotoxicity data. Environ Toxicol Chem 35:1297–1309. https://doi.org/10.1002/etc.3259
Novotný Č, Dias N, Kapanen A, Malachová K, Vándrovcová M, Itävaara M, Lima N (2006) Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 63:1436–1442. https://doi.org/10.1016/J.CHEMOSPHERE.2005.10.002
OECD (2004) Test No. 202: Daphnia sp. acute immobilisation test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264069947-en
OECD (2011) Test No. 201: alga, growth inhibition test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264069923-en
OECD (2012) Test No. 211: Daphnia magna reproduction test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264185203-en
OECD (2013) Test No. 236: Fish embryo acute toxicity (FET) Test. OECD Guidel Test Chem. https://doi.org/10.1787/9789264203709-en
Øllgaard H, Frost L, Galster J, Hansen OC (1998) Survey of azo-colorants in Denmark: consumption, use, health and envi-ronmental aspects
Ozmen N, Erdemoglu S, Gungordu A, Asilturk M, Turhan DO, Akgeyik E, Harper SL, Ozmen M (2018) Photocatalytic degradation of azo dye using core@shell nano-TiO2 particles to reduce toxicity. Environ Sci Pollut Res 25(29):29493–29504
Parrott JL, Bartlett AJ, Balakrishnan VK (2016) Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ Pollut 210:40–47
Rajaguru P, Vidya L, Baskarasethupathi B, Kumar PA, Palanivel M, Kalaiselvi K (2002) Genotoxicity evaluation of polluted ground water in human peripheral blood lymphocytes using the comet assay. Mutat Res 517:29–37
Rocha PS, Bernecker C, Strecker R, Mariani CF, Pompêo MLM, Storch V, Hollert H, Braunbeck T (2011) Sediment-contact fish embryo toxicity assay with Danio rerio to assess particle-bound pollutants in the Tietê River Basin (São Paulo, Brazil). Ecotoxicol Environ Saf 74:1951–1959. https://doi.org/10.1016/j.ecoenv.2011.07.009
Rocha OP, Cesila CA, Christovam EM, Barros SBM, Zanoni MVB, de Oliveira DP (2017) Ecotoxicological risk assessment of the “Acid Black 210” dye. Toxicology 376:113–119. https://doi.org/10.1016/j.tox.2016.04.002
Shen B, Liu H-C, Ou W-B, Eilers G, Zhou SM, Meng FG, Li CQ, Li YQ (2015) Toxicity induced by Basic Violet 14, Direct Red 28 and Acid Red 26 in zebrafish larvae. J Appl Toxicol n/a-n/a 35:1473–1480. https://doi.org/10.1002/jat.3134
Suares-Ruppert EE Zoologia dos Invertebrados - 7aed. 2005.
Tatarazako N, Oda S (2007) The water flea Daphnia magna (Crustacea, Cladocera) as a test species for screening and evaluation of chemicals with endocrine disrupting effects on crustaceans. Ecotoxicology 16:197–203. https://doi.org/10.1007/s10646-006-0120-2
Tatebe C, Zhong X, Ohtsuki T, Kubota H, Sato K, Akiyama H (2014) A simple and rapid chromatographic method to determine unauthorized basic colorants (rhodamine B, auramine O, and pararosaniline) in processed foods. Food Sci Nutr 2:547–556. https://doi.org/10.1002/fsn3.127
Tippabathani J, Nellore J, Kathirkannan P, Nachiyar CV (2020) Developmental effects of three textile chemicals on locomotor activity, antioxidant markers and acetylcholine esterase activity in zebrafish
Tripathi M, Khanna SK, Das M (2007) Surveillance on use of synthetic colours in eatables vis a vis Prevention of Food Adulteration Act of India. Food Control 18:211–219. https://doi.org/10.1016/J.FOODCONT.2005.09.016
Trottier S, Blaise C, Kusui T, Johnson EM (1997) Acute toxicity assessment of aqueous samples using a microplate-basedHydra attenuata assay. Environ Toxicol Water Qual 12:265–271. https://doi.org/10.1002/(SICI)1098-2256(1997)12:3<265::AID-TOX10>3.0.CO;2-9
USEPA (1990) Aerobic and anaerobic treatment of C.I. Disperse Blue 79. 6 pp
Vacchi FI, Albuquerque AF, Vendemiatti JA, Morales DA, Ormond AB, Freeman HS, Zocolo GJ, Zanoni MVB, Umbuzeiro G (2013) Chlorine disinfection of dye wastewater: implications for a commercial azo dye mixture. Sci Total Environ 442:302–309. https://doi.org/10.1016/j.scitotenv.2012.10.019
Vacchi FI, Von der Ohe PC, de Albuquerque AF et al (2016) Occurrence and risk assessment of an azo dye—the case of Disperse Red 1. Chemosphere 156:95–100. https://doi.org/10.1016/j.chemosphere.2016.04.121
Vacchi FI, de Vendemiatti JA, S, da Silva BF et al (2017) Quantifying the contribution of dyes to the mutagenicity of waters under the influence of textile activities. Sci Total Environ 601–602:230–236. https://doi.org/10.1016/j.scitotenv.2017.05.103
Verma Y (2008) Acute toxicity assessment of textile dyes and textile and dye industrial effluents using Daphnia magna bioassay. Toxicol Ind Health 24:491–500. https://doi.org/10.1177/0748233708095769
White CR, Davies SJ, Henry TB (2012) Malachite green toxicity and effects on reproductive success in zebrafish. Zebrafish 9(3):135–139
Wong CK, Liu XJ, Lee AOK, Wong PK (2007) Effect of Azo Dyes on survivorship, oxygen consumption rate, and filtration rate of the freshwater Cladoceran Moina macrocopa. Hum Ecol Risk Assess An Int J 12:289–300. https://doi.org/10.1080/10807030500531604
Zocolo GJ, Pilon dos Santos G, Vendemiatti J, Vacchi FI, Umbuzeiro GA, Zanoni MVB (2015) Using SPE-LC-ESI-MS/MS analysis to assess disperse dyes in environmental water samples. J Chromatogr Sci 53:1–8. https://doi.org/10.1093/chromsci/bmu221
Funding
This study was supported by the Brazilian Ministry of Education, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) (Finance Code 001) through a personal grant provided to CCJA, and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) RO (grant no. 2018/03108-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments are in accordance with the current laws of the country in which they were performed. The study was approved by the Ethics Committee at the University of Brasilia (reference no 100226/2014).
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
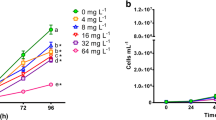
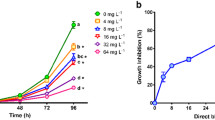
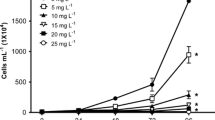
• Assessment of aquatic toxicity of auramine dyes using organisms from different trophic levels, Raphidocelis subcapitata (autotrophs), Dapnhia similis (primary consumers), and Hydra attenuata and Danio rerio (secondary consumers).
• Lethality for D. similis, H. attenuata, and D. rerio in concentrations below of 4800 μg/L.
• Induced inhibition in the growth rate of exposed algae, negatively affecting the reproduction of D. similis and induced deformities in H. attenuata (clubbed tentacles and shortened tentacles) and D. rerio (edemas, tail malformation and delay in yolk sac absorption) in sublethal concentrations.
• PNEC values of 0.92 μg/L (Auramine) and 4.6 μg/L (Auramine O) were derived.
Electronic supplementary material
ESM 1
(DOCX 102 kb)
ESM 2
(DOCX 38087 kb)
Supplemental material SM3A
CRED evaluation Auramine (XLSX 20 kb)
Supplemental material SM3B
CRED evaluation Auramine O (XLSX 21 kb)
Rights and permissions
About this article
Cite this article
de Jesus Azevedo, C.C., de Oliveira, R., Suares-Rocha, P. et al. Auramine dyes induce toxic effects to aquatic organisms from different trophic levels: an application of predicted non-effect concentration (PNEC). Environ Sci Pollut Res 28, 1866–1877 (2021). https://doi.org/10.1007/s11356-020-10462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10462-3