Abstract
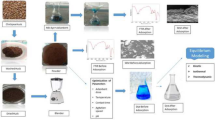
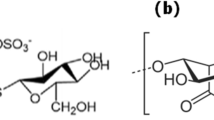
The present study focused on phosphorus adsorption by novel fungal conidiophores biomass in aqueous solution. Fungal Conidiophores biomass was prepared from the fungal strains Aspergillus oryzae (YFK) and Fusarium oxysporum (YVS2). The functional groups and morphology of Conidiophores Biomass (CB) from these strains were characterized by FTIR and SEM. FTIR confirms the presence of alcohol, carboxylic acid, carbon dioxide, cyclic alkene, amine, alkene, fluoro compound, and halo compound groups. Batch mode study was carried out with two CB’s such as Aspergillus oryzae CB (ACB) and Fusarium oxysporum CB (FCB) with initial concentration of phosphorus ranging from 20 to 100 mg L−1. Based on the batch experiments, the adsorption kinetics (pseudo first order and pseudo second order), isotherms (Freundlich and Langmuir models), and thermodynamic (standard entropy, energy, and enthalpy) parameters were calculated. The adsorption kinetics and isotherm studies showed that the adsorption data well fitted with PSO kinetic model. From the isotherm results, it was found that ACB and FCB exhibited highest adsorption capacity 25.64 mg g−1 and 26.32 mg g−1 of phosphorus respectively at the optimal condition of pH (7), time (90 min), dose (250 mg), and room temperature (35 °C). Thermodynamics values were found to be endothermic and spontaneous in nature for phosphorus adsorption. Finally, the results suggested that the ACB and FCB are economically feasible cost-effective adsorbent for removal of phosphorus in wastewater treatment.

Graphical abstract








Similar content being viewed by others
References
Agarwal S, Tyagi I, Gupta VK, Ghasemi N, Shahivand M, Ghasemi M (2016) Kinetics, equilibrium studies and thermodynamics of methylene blue adsorption on Ephedra strobilacea saw dust and modified using phosphoric acid and zinc chloride. J Mol Liq 218:208–218
Antunes E, Schumann J, Brodie G, Jacob MV, Schneider PA (2017) Biochar produced from biosolids using a single-mode microwave: characterization and its potential for phosphorus removal. J Environ Manag 196:119–126
Arwa A, Abeer-Al B, Amani-Al ON-AA, Jh I, Al-T A (2018) Kinetic and thermodynamic study of phosphate removal from water by adsorption onto (Arundodonax) reeds. Adsorpt Sci Technol 36:46–61
Awofolu OR, Mbolekwa Z, Mtshemle V, Fatoki OS (2005) Level of trace metal in water and sediment from Tyume and its effected on an irrigaredFarmland. Water SA 31:87–94
Awuah E, Oppong-Peprah M, Lubberding HJ, Gijzen HJ (2004) Comparative performance studies of water lettuce, duckweed, and algal-based stabilization ponds using low-strength sewage. J Toxicol Environ Health A 67:1727–1739
Beltrame KK, Cazetta AL, de Souza PS, Spessato L, Silva TL, Almeida VC (2018) Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol Environ Saf 147:64–71
Cai R, Wang X, Ji X, Peng B, Tan C, Huang X (2017) Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth. J Environ Manag 187:212–219
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45
Daou TJ, Begin-Colin S, Greneche JM, Thomas F, De-rory A, Bernhardt P, Legare P, Pourroy G (2007) Phosphate adsorption properties of magnetite-based nano-particles. Chem Mater 19:4494–4505
Durairaj K, Velmurugan P, Park JH, Chang WS, Park YJ, Senthilkumar P, Choi KM, Lee JH, Oh BT (2018a) Characterization and assessment of two bio control bacteria against Pseudomonas syringae wilt in Solanumlycopersicum and its genetic responses. Microbiol Res 206:43–49
Durairaj K, Velmurugan P, Vedhanayakisri KA, Chang WS, Senthilkumar P, Choi KM, Lee Oh BT (2018b) Molecular and phenotypic characterization of pathogenic fungal strains isolated from ginseng root rot. Physiol Mol Plant Pathol 104:141–146
Durairaj K, Senthilkumar P, Priya V, Velmurugan P, Jagadeesh Kumar A (2018c) Novel synthesis of Chrysanthemum indicum flower as an adsorbent for the removal of direct Congo red from aqueous solution. Desalin Water Treat 113:270–280
Hall K (2018) Biochar as a sorbent for naturally occurring and synthetic agricultural chemicals. Retrieved from the University of Minnesota Digital Conservancy. http://hdl.handle.net/11299/200178
Jack J, Huggins TM, Huang Y, Fang Y, Ren ZJ (2019) Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J Clean Prod 224:100–106
Jagadeesh Kumar A, Namasivayam C (2014) Uptake of endocrine disruptor Bisphenol-A onto sulphuric acid activated carbon developed from biomass: equilibrium and kinetic studies. Sustain Environ Res 1:24–30
Jagadeesh Kumar A, Prasad Singh R, Fu D, Namasivayam C (2017a) Comparison of physical- and chemical-activated Jatropha Curcas husk carbon as an adsorbent for the adsorption of Reactive Red 2 from aqueous solution. Desalin Water Treat 95:308–318
Jagadeesh Kumar A, Prasad Singh R, Fu D, Namasivayam C (2017b) Low cost meso-porous carbon developed from Jatropha husk, a bio-diesel byproduct waste for adsorption of methylene blue. Fresenius Environ Bull 26:5723–5731
Jain P, Kichi DS (2014) Phosphate solubilizing microorganism (psm): an ecofriendly biofertilizer and pollution manager. J Dyn Agric Res 1:23–28
Jeppu GP, Clement TP (2012) A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J Contam Hydrol 129:46–53
John Y, David VE, Mmereki D (2018) A comparative study on removal of hazardous anions from water by adsorption: a review. Int J Chem Eng. https://doi.org/10.1155/2018/3975948
Jung KW, Lee S, Lee Y (2017) Synthesis of novel magnesium ferrite (MgFe2O4) biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour Technol 245:751–759
Kalaimurugan D, Sivasankar P, Manikandan E, Durairaj K, Lavanya K, Vasudhevan P, Lakshmanan R, Venkatesan S (2019a) Spatial determination of soil variables using GIS method and their influence on microbial communities in the eastern Ghats region. Trop Ecol 1:14
Kalaimurugan D, Sivasankar P, Lavanya K, Shivakumar MS, Venkatesan S (2019b) Antibacterial and Larvicidal activity of Fusarium proliferatum (YNS2) whole cell biomass mediated copper nanoparticles. J Clust Sci 30(4):1071–1080
Karaca S, Gurses A, Ejder M, Acikyildiz M (2004) Kinetic modeling of liquid-phase adsorption of phosphate on dolomite. J Colloid Interface Sci 277:257–263
Kaveeshwar AR, Ponnusamy SK, Revellame ED, Gang DD, Zappi ME, Subramaniam R (2018) Pecan shell based activated carbon for removal of iron (II) from fracking wastewater: adsorption kinetics, isotherm and thermodynamic studies. Process Saf Environ Prot 114:107–122
Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosynthesis 41(1–2):197–207
Kim J, Li W, Philips BL, Grey CP (2011) Phosphate adsorption on the iron oxyhydroxides goethite (α-FeOOH), akaganeite (β-FeOOH), and lepidocrocite (γ-FeOOH): a 31 P NMR study. Energy Environ Sci 4(10):4298–4305
Li X, Xu H, Gao B, Shi X, Sun Y, Wu J (2018) Efficient biosorption of Pb(II) from aqueous solutions by a PAH-degrading strain Herbaspirillum chlorophenolicum FA1. J Ind Eng Chem 57:64–71
Liu X, Zhang L (2015) Removal of phosphate anions using the modified chitosan beads: adsorption kinetic, isotherm and mechanism studies. Powder Technol 277:112–119
Liu T, Li H, Li Z, Xiao X, Chen L, Deng L (2007) Removal of hexavalent chromium by fungal biomass of Mucor racemosus: influencing factors and removal mechanism. World J Microbiol Biotechnol 23(12):1685–1693
Mahardika D, Park HS, Choo KH (2018) Ferrihydrite-impregnated granular activated carbon (FH@ GAC) for efficient phosphorus removal from wastewater secondary effluent. Chemosphere 207:527–533
Mezenner NY, Bensmaili A (2009) Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem Eng J 147:87–96
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53
Moharami S, Jalali M (2013) Removal of phosphorus from aqueous solution by Iranian natural adsorbents. Chem Eng J 223:328–339
Mor S, Chhoden K, Ravindra K (2016) Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J Clean Prod 129:673–680
Mor S, Chhoden K, Negi P, Ravindra K (2017) Utilization of nano-alumina and activated charcoal for phosphate removal from wastewater. Environ Nanotech Monit Manag 7:15–23
Mulkerrins DDE, Dobson ADW, Colleran E (2004) Parameters affecting biological phosphate removal from wastewaters. Envi inter 30(2):249–259
Naghibi H, Tamura A, Sturtevant JM (1995) Significant discrepancies between van't Hoff and calorimetric enthalpies. Proc Natl Acad Sci 92:5597–5599
Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM (2002) Thermo tolerance generated by plant/fungal symbiosis. Sci 298:1581
Ren J, Li N, Li L, An JK, Zhao L, Ren NQ (2015) Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour Technol 178:119–125
Rezaee A, Derayat J, Mortazavi SB, Yamini Y, Jafarzadeh MT (2005) Removal of mercury from chlor-alkali industry wastewater using Acetobacter xylinum cellulose. Am J Environ Sci 1(2):105
Rodrigues LA, Silva ML (2010) Thermodynamic and kinetic investigations of phosphate adsorption onto hydrous niobium oxide prepared by homogeneous solution method. Desalination 263:29–35
Rodríguez ME, Miranda RC, Olivas R, Sosa CA (2008) Efectos de las condiciones de operación sobre la biosorción de Pb2+, Cd2+ y Cr3+ en solución por Saccharomyces cerevisiae residual. Inf Tecnol 19(6):47–55
Rodriguez RJ, White JJF, Arnold AE, Redman RS (2009) Fungal entophytes: diversity and functional roles. New Phytol 182:314–330
Safa Y (2016) Utilization of mustard and linseed oil cakes: novel biosorbents for removal of acid dyes. Desalin Water Treat 57(13):5914–5925
Safa A-S, Andrew K, Claire T, Bradley S, Satomi M, Istvan B, Boris R, Al A, Tibor H, Christopher ES (2011) P62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122:691–702
Salama RS, Kiwaan H, Mostafa MR (2016) Remediating free chlorine from aqueous solution using hydrous zirconium oxide impregnated carbons. J Chem Eng Process Technol 7:3
Sikora RA, Pocasangre L, Felde AZ, Niere B, Vu TT, Dababat AA (2008) Mutualistic endophytic fungi and in-plantaSuppressiveness to plant parasitic nematodes. Biol Control 46:15–23
Silva TL, Cazetta AL, Souza PS, Zhang T, Asefa T, Almeida VC (2018) Mesoporous activated carbon fibers synthesized from denim fabric waste: efficient adsorbents for removal of textile dye from aqueous solutions. J Clean Prod 171:482–490
Sleiman N, Deluchat V, Wazne M, Courtin A, Saad Z, Kazpard V, Baudu M (2016) Role of iron oxidation byproducts in the removal of phosphate from aqueous solution. RSC Adv 6(2):1627–1636
Standard DI (1989) Bureau of Indian standards protocol
Supriya S, Palanisamy PN, Shanthi P (2014) Preparation and characterization of activated carbon from Casuarina for the removal of dyes from textile wastewater. Int J Chem Tech Res 6(7):3635–3641
Wang DB, Yang GJ, Li XM, Zheng W, Wu Y, Yang Q, Zeng GM (2012) Inducing mechanism of biological phosphorus removal driven by the aerobic/extended-idle regime. Biotechnol Bioeng 109:2798–2807
Wang N, Jin RN, Omer AM, Ouyang XK (2017) Adsorption of Pb(II) from fish sauce using carboxylated cellulose nanocrystal: isotherm, kinetics, and thermodynamic studies. Int J Biol Macromol 102:232–240
Xiong W, Tong J, Yang Z, Zeng G, Zhou Y, Wang D, Song P, Xu R, Zhang C, Cheng M (2017) Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon Nano fiber: performance and mechanism. J Colloid Interface Sci 493:17–23
Yang Q, Wang X, Luo W, Sun J, Xu Q, Chen F, Zhao J, Wang S, Yao F, Wang D, Li X (2018) Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour Technol 247:537–544
Yao H, Lu J, Wu J, Lu Z, Wilson PC, Shen Y (2013) Adsorption of fluoroquinolone antibiotics by wastewater sludge biochar: role of the sludge source. Water, Air, & Soil Pollution 224(1):1370
Yu XZ, Feng YX, Liang YP (2018) Kinetics of phyto-accumulation of hexavalent and trivalent chromium in rice seedlings. Int Biodeterior Biodegrad 128:72–77
Zhang XH, Yu XZ, Liang YP (2014) Parameter determination involved in phytotoxicity and transport of cadmium in rice seedlings. Int Biodeterior Biodegrad 96:121–126
Zhang J, Bligh MW, Liang P, Waite TD, Huang X (2018) Phosphorus removal by in situ generated Fe (II): efficacy, kinetics and mechanism. Water Res 136:120–130
Acknowledgments
D. K. is thankful to Periyar University for University Research Fellowship and K. D. is gratefully acknowledge to Council of Scientific and Industrial Research (CSIR), New Delhi, for providing financial assistance (Award No: 09/810/0025/2018 EMR-I) in the form of Senior Research Fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Efficient and ecofriendly adsorbent for phosphorus removal using fungi.
• Aspergillus oryzae conidiophores biomass and Fusarium oxysporum conidiophores biomass fungal strains adsorb more phosphorus at temperature (35 °C) and pH (7).
• FTIR Functional groups confirms the presence of alcohol, carboxylic acid, carbon dioxide, cyclic alkene, amine, alkene, fluoro compound, and halo compounds.
• Phylogenetic tree analysis for fungal strain in FCB and ACB Gen Bank with Accession no. of MH539641 and MH539642.
Rights and permissions
About this article
Cite this article
Kalaimurugan, D., Durairaj, K., Kumar, A.J. et al. Novel preparation of fungal conidiophores biomass as adsorbent for removal of phosphorus from aqueous solution. Environ Sci Pollut Res 27, 20757–20769 (2020). https://doi.org/10.1007/s11356-020-08307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08307-0