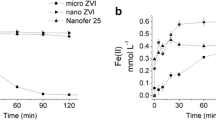
Abstract
Conventional wastewater treatments are not efficient in removing parabens, which may thus end up in surface waters, posing a threat to aquatic biota and human health. As an alternative treatment, persulfate (PS)-driven advanced oxidation technologies have gained growing attention for removing these pollutants. In this study, the degradation of propylparaben (PrP) by UVA- and zero-valent iron (ZVI)-activated persulfate was investigated. The effects of initial PS concentration ([PS]0) and irradiance or ZVI concentration were explored using the Doehlert experimental design. For the UVA-activated system, the specific PrP degradation rate (k) and percent removal were consistently higher for increasing [PS]0 and irradiance, varying in the ranges 0.0053–0.0192 min−1 and 37.9–77.3%, respectively. In contrast, extremely fast PrP degradation was achieved through the ZVI/PS process (0.3304 < k < 0.9212 min−1), with removal percentages above 97.5%; in this case, paraben degradation was hindered for a ZVI dosage beyond 40 mg L−1. Regarding toxicity, ECOSAR predictions suggest that the degradation products elucidated by LC-MS/MS are less toxic than PrP toward fish, daphnid, and green algae. In addition, both processes showed to be strongly dependent on the water matrix, being ZVI/PS more impacted for a MBR effluent, although its performance was much better than that exhibited by the UVA-driven process (t1/2 of 65.4 and 276.1 min, respectively).









Similar content being viewed by others
References
An J, Xia C, He J, Feng H (2018) Oxidation of propyl paraben by ferrate (VI): kinetics, products, and toxicity assessment. J Environ Sci Health A 53:873–882
Andersen FA (2008) Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, and benzylparaben as used in cosmetics products. Int J Toxicol 27:1–82
Bazin I, Gadal A, Touraud E, Roig B (2010) Hydroxy benzoate preservatives (parabens) in the environment: data for environmental toxicity assessment. Environ Pollut 16:245–257
Bekris L, Frontistis Z, Trakakis G, Sygellou L, Galiotis C, Mantzavinos D (2017) Graphene: a new activator of sodium persulfate for the advanced oxidation of parabens in water. Water Res 126:111–121
Bennedsen LR, Muff J, Sogaard EG (2012) Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 86:1092–1097
Błedzka D, Gromadzińska J, Wasowicz W (2014) Parabens. From environmental studies to human health. Environ Int 67:27–42
Brasil (2005) CONAMA Resolution 357. Published in Official Gazette 053 on March 18, 2005, page 58
Brasil (2011) CONAMA Resolution 430. Published in Official Gazette 092 on May 16, 2011, page 89
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. Atomic Energy 17:513–886
Chen Y, Deng P, Xie P, Shang R, Wang Z, Wang S (2017) Heat-activated persulfate oxidation of methyl- and ethyl-parabens: effect, kinetics, and mechanism. Chemosphere 168:1628–1636
Darbre PD (2003) Underarm cosmetics and breast cancer. J Appl Toxicol 23:89–95
Darbre PD, Aljarrah A, Miller WR, Coldham NG, Sauer MJ, Pope GS (2004) Concentrations of parabens in human breast tumours. J Appl Toxicol 24:5–13
Dhaka S, Kumar R, Khan MA, Paeng KJ, Kurade MB, Kim SJ, Jeon BH (2017) Aqueous phase degradation of methyl paraben using UV-activated persulfate method. Chem Eng J 321:11–19
Dhaka S, Kumar R, Lee SH, Kurade MB, Jeon BH (2018) Degradation of ethylparaben in aqueous medium using advanced oxidation processes: efficiency evaluation of UV-C supported oxidants. J Clean Prod 180:505–513
Doehlert DH (1970) Uniform shell designs. J R Stat Soc: Ser C: Appl Stat 19:231–239
Ferreira SLC, Santos WNL, Quintella CM, Neto BB, Bosque-Sendra JM (2004) Doehlert matrix: a chemometric tool for analytical chemistry – review. Talanta 63:1061–1067
Fogler HS (2006) Elements of chemical reaction engineering, 4th edn. Pearson Education, Pennsylvania
Foszpańczyk M, Bednarczyk K, Drozdek E, Martins RC, Ledakowicz S, Gmurek M (2018) Comparison of photocatalytic and photosensitized oxidation of paraben aqueous solutions under sunlight. Water Air Soil Pollut 229:362
Frontistis Z, Antonopoulou M, Konstantinou I, Mantzavinos D (2017) Degradation of ethyl paraben by heat-activated persulfate oxidation: statistical evaluation of operating factors and transformation pathways. Environ Sci Pollut Res 24:1073–1084
Ghauch A, Ayoub G, Naim S (2013) Degradation of sulfamethoxazole by persulfate assisted micrometric Fe0 in aqueous solution. Chem Eng J 228:1168–1181
Gmurek M, Rossi AF, Martins RC, Quinta-Ferreira R, Ledakowick S (2015) Photodegradation of single and mixture of parabens – kinetic, by-products identification and cost-efficiency analysis. Chem Eng J 276:03–314
Graça CAL, Velosa AC, Teixeira ACSC (2017) Role of Fe(III)-carboxylates in AMZ photodegradation: a response surface study based on a Doehlert experimental design. Chemosphere 184:981–991
Graça CAL, Fugita LTN, Velosa AC, Teixeira ACSC (2018) Amicarbazone degradation promoted by ZVI-activated persulfate: study of relevant variables for practical application. Environ Sci Pollut Res 25:5474–5483
Graça CAL, Maniero MG, Andrade LM, Guimarães JR, Teixeira ACSC (2019) Evaluation of amicarbazone toxicity removal through degradation processes based on hydroxyl and sulfate radicals. J Environ Sci Health 54:1126–1143
Han D, Wan J, Ma Y, Wang Y, Li Y, Li D, Guan Z (2015) New insights into the role of organic chelating agents in the Fe(II) activated persulfate processes. Chem Eng J 269:425–433
Ioannidi A, Frontistis Z, Mantzavinos D (2018) Destruction of propyl paraben by persulfate activated with UV-A light emitting diodes. J Environ Chem Eng 6:2992–2997
Liang C, Liang C-P, Chen C-C (2009) pH dependence of persulfate activation by EDTA/Fe(III) for degradation of trichloroethylene. J Contam Hydrol 106:173–182
Mayo-Bean K, Moran K, Meylan B, Ranslow P (2012) Methodology document for the ecological structure-activity relationship model (ECOSAR) class program. US-EPA, Washington D.C
Metheneti ME, Frontistis Z, Ribeiro RS, Silva AMT, Faria JL, Gomes HT, Mantzavinos D (2017) Degradation of propylparaben by activated persulfate using iron-containing magnetic carbon xerogels: investigation of water matrix and process synergy effects. Environ Sci Pollut Res 25:34801–34810
Neta P, Huie RE, Ross AB (1988) Rate constants for reaction of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284
Oppenländer T (2003) Photochemical purification of water and air: advanced oxidation processes (AOPs): principles, reaction mechanisms, reactor concepts. Wiley-VCH
Pipolo M, Gmurek M, Corceiro V et al (2017) Ozone-based technologies for parabens removal from water: toxicity assessment. Ozone Sci Eng 39:233–243
Souza LP, Graça CAL, Taqueda MES, Teixeira ACSC, Chiavone-Filho O (2019) Insights into the reactivity of zero-valent-copper-containing materials as reducing agents of 2,4,6-trichlorophenol in a recirculation packed-column system: degradation mechanism and toxicity evaluation. Process Saf Environ 127:348–358
Tay KA, Rahman NA, Abas MRB (2010) Ozonation of parabens in aqueous solution: kinetics and mechanism of degradation. Chemosphere 81:1446–1453
Wei X, Gao N, Li C, Deng Y, Zhou S, Li L (2016) Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem Eng J 285:660–670
Funding
The authors express their gratitude to the Brazilian National Council for Scientific and Technological Development (CNPq, grant no.131467/2017-4) and to the São Paulo Research Foundation (FAPESP, grant no. 2013/50218-2) for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1409 kb)
Rights and permissions
About this article
Cite this article
Palharim, P.H., Graça, C.A.L. & Teixeira, A.C.S.C. Comparison between UVA- and zero-valent iron-activated persulfate processes for degrading propylparaben. Environ Sci Pollut Res 27, 22214–22224 (2020). https://doi.org/10.1007/s11356-020-08141-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08141-4