Abstract
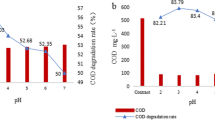
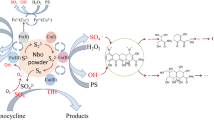
Alarming amounts of organic pollutants are being detected in waterbodies due to their ineffective removal by conventional treatment techniques, which warn of the urgent need of developing new technologies for their remediation. In this context, advanced oxidation processes (AOPs), especially those based on Fenton reactions, have proved to be suitable alternatives, due to their efficacy of removing persistent organic compounds. However, the use of ferrous iron in these processes has several operational constraints; to avoid this, an alternative iron source was here investigated: zero-valent-iron (ZVI). A Fenton-like process based on the activation of a recently explored oxidant-persulfate (PS)—with ZVI was applied to degrade an emerging contaminant: Amicarbazone (AMZ). The influence of ZVI size and source, PS/ZVI ratio, pH, UVA radiation, dissolved O2, and inorganic ions was evaluated in terms of AMZ removal efficiency. So far, this is the first time these parameters are simultaneously investigated, in the same study, to evaluate a ZVI-activated PS process. The radical mechanism was also explored and two radical scavengers were used to determine the identity of major active species taking part in the degradation of AMZ. The degradation efficiency was found to be strongly affected by the ZVI dosage, while positively affected by the PS concentration. The PS/ZVI system enabled AMZ degradation in a wide range of pH, although with a lower efficiency under slightly alkaline conditions. Dissolved O2 revealed to play an important role in reaction kinetics as well as the presence of inorganic ions. UVA radiation seems to improve the degradation kinetics only in the presence of extra O2 content. Radicals quenching experiments indicated that both sulfate (SO4 •−) and hydroxyl (•OH) radicals contributed to the overall oxidation performance, but SO4 •− was the dominant oxidative species.








Similar content being viewed by others
References
Avetta P, Pensato A, Minella M, Malandrino M, Maurino V, Minero C, Hanna K, Vione D (2015) Activation of persulfate by irradiated magnetite: implications for the degradation of phenol under heterogeneous photo-fenton-like conditions. Environ Sci Technol 49(2):1043–1050. https://doi.org/10.1021/es503741d
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2(1):557–572. https://doi.org/10.1016/j.jece.2013.10.011
Bennedsen LR, Muff J, Søgaard EG (2012) Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 86(11):1092–1097. https://doi.org/10.1016/j.chemosphere.2011.12.011
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J Phys Chem Ref Data 17(2):513–886. https://doi.org/10.1063/1.555805
Cao JS, Zhang WX, Brown DG, Sethi D (2008) Oxidation of lindane with Fe(II)-activated sodium persulfate. Environ Eng Sci 25(2):221–228. https://doi.org/10.1089/ees.2006.0244
Cieśla P, Kocot P, Mytych P, Stasicka Z (2004) Homogeneous photocatalysis by transition metal complexes in the environment. J Mol Catal A Chem 224(1-2):17–33. https://doi.org/10.1016/j.molcata.2004.08.043
Conceição DS, Ferreira DP, Graça CAL, Júlio MF, Ilharco LM, Velosa AC, Santos PF, Vieira Ferreira LF (2017) Photochemical and photocatalytic evaluation of 1D titanate / TiO2 based nanomaterials. Appl Surf Sci 392:418–429. https://doi.org/10.1016/j.apsusc.2016.09.067
Correia de Velosa A, Pupo Nogueira RF (2013) 2,4-Dichlorophenoxyacetic acid (2,4-D) degradation promoted by nanoparticulate zerovalent iron (nZVI) in aerobic suspensions. J Environ Manag 121:72–79. https://doi.org/10.1016/j.jenvman.2013.02.031
Deng J, Shao Y, Gao N, Deng Y, Tan C, Zhou S (2014) Zero-valent iron/persulfate(Fe0/PS) oxidation acetaminophen in water. Int J Environ Sci Technol 11(4):881–890. https://doi.org/10.1007/s13762-013-0284-2
Eberhardt M (2001) Reactive oxygen metabolites. In: Chemistry and Medical Consequences. CRC Press, Boca Raton
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205. https://doi.org/10.1016/j.jhazmat.2013.12.062
Furman OS, Teel AL, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44(16):6423–6428. https://doi.org/10.1021/es1013714
Gao YQ, Gao NY, Deng Y et al (2012) Ultraviolet (UV) light-activated persulfate oxidation of sulfamethazine in water. Chem Eng J 195–196:248–253. https://doi.org/10.1016/j.cej.2012.04.084
Ghauch A, Ayoub G, Naim S (2013) Degradation of sulfamethoxazole by persulfate assisted micrometric Fe0 in aqueous solution. Chem Eng J 228:1168–1181. https://doi.org/10.1016/j.cej.2013.05.045
Graça CAL, Velosa AC, Teixeira ACSC (2017a) Amicarbazone degradation by UVA-activated persulfate in the presence of hydrogen peroxide or Fe2+. Catal Today 280:80–85. https://doi.org/10.1016/j.cattod.2016.06.044
Graça CAL, Velosa AC, Teixeira ACSC (2017b) Role of Fe(III)-carboxylates in AMZ photodegradation: a response surface study based on a Doehlert experimental design. Chemosphere 184:981–991. https://doi.org/10.1016/j.chemosphere.2017.06.013
Han D, Wan J, Ma Y, Wang Y, Li Y, Li D, Guan Z (2015) New insights into the role of organic chelating agents in Fe(II)-activated persulfate processes. Chem Eng J 269:425–433. https://doi.org/10.1016/j.cej.2015.01.106
Ji Y, Dong C, Kong D, Lu J, Zhou Q (2015) Heat-activated persulfate oxidation of atrazine: implications for remediation of groundwater contaminated by herbicides. Chem Eng J 263:45–54. https://doi.org/10.1016/j.cej.2014.10.097
Li H, Wan J, Ma Y, Wang Y, Huang M (2014) Influence of particle size of zero-valent iron and dissolved silica on the reactivity of activated persulfate for degradation of acid orange 7. Chem Eng J 237:487–496. https://doi.org/10.1016/j.cej.2013.10.035
Li H, Wan J, Ma Y, Wang Y, Guan Z (2015) Role of inorganic ions and dissolved natural organic matters on persulfate oxidation of acid orange 7 with zero-valent iron. RSC Adv 5(121):99935–99943. https://doi.org/10.1039/C5RA16094D
Liang C, Wang Z, Bruell CJ (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66(1):106–113. https://doi.org/10.1016/j.chemosphere.2006.05.026
Liu CS, Shih K, Sun CX, Wang F (2012) Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci Total Environ 416:507–512. https://doi.org/10.1016/j.scitotenv.2011.12.004
Ma Y-S, Sung C-F (2010) Investigation of carbofuran decomposition by a combination of ultrasound and Fenton process. J Environ Eng Manag 20:213–219
MacKul’Ak T, Prousek J, Švorc L (2011) Degradation of atrazine by Fenton and modified Fenton reactions. Monatshefte fur Chemie 142(6):561–567. https://doi.org/10.1007/s00706-011-0504-8
Mortatti FJ, Krug LCR, Pessenda et al (1982) Determination of iron in natural waters and plant material with 1,10-phenanthroline by flow injection analysis. Analyst 107(659–6):659–663. https://doi.org/10.1039/AN9820700659
Neta P, Huie RE, Ross AB (1988) Rate constants for reaction of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284
Oh SY, Kim HW, Park JM et al (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168(1):346–351. https://doi.org/10.1016/j.jhazmat.2009.02.065
Oppenländer T (2003) Photochemical purification of water and air: advanced oxidation processes (AOPs): principals, reaction mechanisms, reactor concepts. Wiley-VCH, Weinheim
Peixoto AL, Teixeira ACSC (2014) Degradation of amicarbazone herbicide by photochemical processes. J Photochem Photobiol A Chem 275:54–64. https://doi.org/10.1016/j.jphotochem.2013.10.013
Phenrat T, Long TC, Lowry GV (2009) Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ Sci Technol 43(1):195–200. https://doi.org/10.1021/es801955n
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34(12):2564–2569. https://doi.org/10.1021/es9911420
Possamai ACS, Inoue MH, Mendes KF, Santana DC, Ben R, Santos EG (2013) Leaching potential and residual effect of amicarbazone in soils of contrasting texture. Semin. Ciênc Agrar 34(5):2203–2210. https://doi.org/10.5433/1679-0359.2013v34n5p2203
Rao YF, Qu L, Yang H, Chu W (2014) Degradation of carbamazepine by Fe(II)-activated persulfate process. J Hazard Mater 268:23–32. https://doi.org/10.1016/j.jhazmat.2014.01.010
Rasoulifard M, Majidzadeh H, Demneh F, Babaei E (2012) Photocatalytic degradation of tylosin via ultraviolet-activated persulfate in aqueous solution. Int J Ind Chem 3(1):16. https://doi.org/10.1186/2228-5547-3-16
Santos EA, Correia NM (2015) Herbicide detection in groundwater in Córrego Rico-SP. Planta Daninha 33:147–155. https://doi.org/10.1590/S0100-83582015000100017
Silva MP, Mostafa S, McKay G, Rosario-Ortiz FL, Teixeira ACSC (2015) Photochemical fate of amicarbazone in aqueous media: laboratory measurement and simulations. Environ Eng Sci 32(8):1–11. https://doi.org/10.1089/ees.2015.0127
Sohrabi MR, Khavaran A, Shariati S, Shariati S (2014) Removal of Carmoisine edible dye by Fenton and photo Fenton processes using Taguchi orthogonal array design. Arab J Chem 10:S3523–S3531. https://doi.org/10.1016/j.arabjc.2014.02.019
Stefan MI (ed) (2017) Advanced oxidation processes for water treatment: fundamentals and applications. IWA publishing, London
Stefaniuk M, Oleszczuk P, Ok Y (2016) Review on nano zerovalent iron (nZVI): from modification to environmental applications. Chem Eng J 287:618–632. https://doi.org/10.1016/j.cej.2015.11.046
Trovó AG, Nogueira RFP, Agüera A, Fernandez-Alba AR, Sirtori C, Malato S (2009) Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res 43(16):3922–3931. https://doi.org/10.1016/j.watres.2009.04.006
Trovó AG, Pupo Nogueira RF, Aguera A et al (2011) Degradation of the antibiotic amoxicillin by photo-Fenton process - chemical and toxicological assessment. Water Res 45(3):1394–1402. https://doi.org/10.1016/j.watres.2010.10.029
Velosa AC, Nascimento CAO (2017) Evaluation of sulfathiazole degradation by persulfate in Milli-Q water and in effluent of a sewage treatment plant. Environ Sci Pollut Res 24(7):6270–6277. https://doi.org/10.1007/s11356-016-7036-z
Wei X, Gao N, Li C, Deng Y, Zhou S, Li L (2016) Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem Eng J 285:660–670. https://doi.org/10.1016/j.cej.2015.08.120
Weng C, Tao H (2015) Highly efficient persulfate oxidation process activated with Fe0 aggregate for decolorization of reactive azo dye Remazol golden yellow. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.05.012
Xu XR, Li XY, Li XZ, Bin LH (2009) Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2 and UV/Fe2+/H2O2 processes. Sep Purif Technol 68(2):261–266. https://doi.org/10.1016/j.seppur.2009.05.013
Zakowski K, Narozny M, Szocinski M (2014) Influence of water salinity on corrosion risk — the case of the southern Baltic Sea coast. Environ Monit Assess 186(8):4871–4879. https://doi.org/10.1007/s10661-014-3744-3
Zhang Q, Chen J, Dai C, Zhang Y, Zhou X (2014) Degradation of carbamazepine and toxicity evaluation using the UV/persulfate process in aqueous solution. J Chem Technol Biotechnol 90(4):701–708. https://doi.org/10.1002/jctb.4360
Acknowledgements
The authors would like to thank Taniele Araújo (from UFRN, Brazil) for her help in some experiments, as well as BE Mundus organization, FAPESP (grant nos. 2013/50218-2, 2013/09543-7, 2013/04656-8) and CAPES-PROCAD (grant 88881.068433/2014-01) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Electronic supplementary material
Figure S1
SEM-FEG image [× 50,000] of synthesized nZVI particles. (JPEG 499 kb)
Rights and permissions
About this article
Cite this article
Graça, C.A.L., Fugita, L.T.N., de Velosa, A.C. et al. Amicarbazone degradation promoted by ZVI-activated persulfate: study of relevant variables for practical application. Environ Sci Pollut Res 25, 5474–5483 (2018). https://doi.org/10.1007/s11356-017-0862-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0862-9