Abstract
The efficiency of the proteolytic strain Anoxybacillus kamchatkensis M1V in the fermentation of speckled shrimp by-product was investigated for the recovery of a deproteinized bioactive hydrolysate. The biological activities of the resulting hydrolysate were also examined by applying several antioxidant and enzyme inhibitory assays. The strain M1V was found to produce high level of protease activity (2000 U/mL) when grown in media containing only shrimp powder at 25 g/L. The crude protease displayed a significant deproteinization capabiliy, with the best efficiency (48%) being recorded for an enzyme to substrate (E/S) ratio of 30 U/mg. Following the deproteinization, chitin was recovered and the authenticity was confirmed by Fourier-transform infrared spectroscopy (FTIR) analysis. On the other hand, the obtained hydrolysate showed a significant enzymatic inhibitory potential against acetylcholinesterase, tyrosinase, amylase, and angiotensin I convertase, and a strong antioxidant activity.

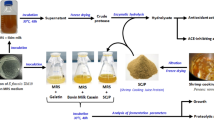
Graphical Abstract




Similar content being viewed by others
Data availability
No data were used to support this study.
References
Abdelhedi O, Jridi M, Jemil I, Mora L, Toldrá F, Aristoy MC, Boualga A, Nasri M, Nasri R (2016) Combined biocatalytic conversion of smooth hound viscera: protein hydrolysates elaboration and assessment of their antioxidant, anti-ACE and antibacterial activities. Food Res Int 86:9–23
Abdelhedi O, Nasri R, Jridi M, Mora L, Oseguera Toledo ME, Aristoy MC, Ben Amara I, Toldrá F, Nasri M (2017) In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: enzyme-peptide interaction study using molecular docking simulation. Process Biochem 58:145–159
Alemán A, Pérez Santín E, Bordenave Juchereau S, Arnaudin I, Gómez Guillén M, Montero P (2011) Squid gelatin hydrolysates with antihypertensive, anticancer, and antioxidant activity. Food Res Int 44:1044–1051
Ansari FA, Khan AA, Mahmood R (2018) Protective effect of carnosine and N-acetylcysteine against sodium nitrite-induced oxidative stress and DNA damage in rat intestine. Environ Sci Pollut Res 25:19380–19392
Arise RO, Yekeen A, Ekun O (2016) In vitro antioxidant and α-amylase inhibitory properties of watermelon seed protein hydrolysates. Environ Exp Biol 14:163–172
Ben Braïek O, Merghni A, Smaoui S, Mastouri M (2019) Enterococcus lactis Q1 and 4CP3 strains from raw shrimps: potential of antioxidant capacity and anti-biofilm activity against methicillin-resistant Staphylococcus aureus strains. J Food Sci Technol 102:15–21
Ben Meriem S (1996) Mortalités (F et M) et analyse des rendements par recrue de Penaeus kerathurus (Forskal, 1775) du golfe de Gabès, Tunisie. Crustacean 66:25–33
Ben Slima S, Ktari N, Trabelsi I, Moussa H, Makni I, Ben Salah R (2018) Purification, characterization and antioxidant properties of a novel polysaccharide extracted from Sorghum bicolor (L.) seeds in sausage. Int J Biol Macromol 106:168–178
Bhagwat PK, Bhise KK, Bhuimbar MV, Dandge PB (2018) Use of statistical experimental methods for optimization of collagenolytic protease production by Bacillus cereus strain SUK grown on fish scales. Environ Sci Pollut Res 25:28226–28236
Bohacz J (2019) Changes in mineral forms of nitrogen and sulfur and enzymatic activities during composting of lignocellulosic waste and chicken feathers. Environ Sci Pollut Res 26:10333–10342
Boudaya L, Mosbahi N, Dauvin JC, Neifar L (2019) Structure of the benthic macrofauna of an anthropogenic influenced area: Skhira Bay (Gulf of Gabès, central Mediterranean Sea). Environ Sci Pollut Res 26:13522–13538
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Colin N, Villéger S, Wilkes M, de Sostoa A, Maceda Veiga A (2018) Functional diversity measures revealed impacts of non-native species and habitat degradation on species-poor freshwater fish assemblages. Sci Total Environ 625:861–871
Cushman D, Cheung H (1971) Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol 20:1637–1648
Dinis LT, Oliveira MM, Almeida J, Costa R, Gomes Laranjo J, Peixoto F (2012) Antioxidant activities of chestnut nut of Castanea sativa Mill. (cultivar ‘Judia’) as function of origin ecosystem. Food Chem 132:1–8
Djellouli M, López Caballero ME, Arancibia MY, Karam N, Martínez Alvarez O (2019) Antioxidant and antimicrobial enhancement by reaction of protein hydrolysates derived from shrimp by-products with glucosamine. Waste Biomass Valori, In press. https://doi.org/10.1007/s12649-019-00607-y
Doan CT, Tran TN, Vo TPK, Nguyen AD, Wang SL (2019) Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int J Biol Macromol 131:706–715
Dubois J (1966) Influence of certain recent modifications in the sugar-cane culture on the evolution of populations of Yanga-guttulata-Sign (French). Tananarive: IRAM, 52 p. (Document IRAM, n. 63). Agron. Trop. (Paris) 21 (6/7) , 786–821 , MAP . Jun/Jul 66. https://agritrop.cirad.fr/368512/
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fields K, Falla T, Rodan K, Bush L (2009) Bioactive peptides: signaling the future. J Cosmet Dermatol 8:8–13
Folch J, Lees M, Sloane Stanley G (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Ghasemi S, Khoshgoftarmanesh AH, Afyuni M, Hadadzadeh H (2014) Iron (II)-amino acid chelates alleviate salt-stress induced oxidative damages on tomato grown in nutrient solution culture. Sci Hortic 165:91–98
Ghorbel Bellaaj O, Hmidet N, Jellouli K, Younes I, Maâlej H, Hachicha R, Nasri M (2011) Shrimp waste fermentation with Pseudomonas aeruginosa A2: optimization of chitin extraction conditions through Plackett-Burman and response surface methodology approaches. Int J Biol Macromol 48:596–602
Ghorbel Bellaaj O, Younes I, Maâlej H, Hajji S, Nasri M (2012) Chitin extraction from shrimp shell waste using Bacillus bacteria. Int J Biol Macromol 51:1196–1201
Guo N, Sun J, Zhang Z, Mao X (2019) Recovery of chitin and protein from shrimp head waste by endogenous enzyme autolysis and fermentation. J Ocean U China 18:719–726
Hajji S, Ghorbel Bellaaj O, Younes I, Jellouli K, Nasri M (2015) Chitin extraction from crab shells by Bacillus bacteria: biological activities of fermented crab supernatants. Int J Biol Macromol 79:167–173
Hamdi M, Hammami A, Hajji S, Jridi M, Nasri M, Nasri R (2017) Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int J Biol Macromol 101:455–463
Hamdi M, Nasri R, Dridi N, Li S, Nasri M (2020) Development of novel high-selective extraction approach of carotenoproteins from blue crab (Portunus segnis) shells, contribution to the qualitative analysis of bioactive compounds by HR-ESI-MS. Food Chem 302:125334
Hamdi M, Nasri R, Li S, Nasri M (2019) Bioactive composite films with chitosan and carotenoproteins extract from blue crab shells: biological potential and structural, thermal, and mechanical characterization. Food Hydrocoll 89:802–812
Hammami A, Hamdi M, Abdelhedi O, Jridi M, Nasri M, Bayoudh A (2017) Surfactant- and oxidant-stable alkaline proteases from Bacillus invictae: characterization and potential applications in chitin extraction and as a detergent additive. Int J Biol Macromol 96:272–281
Holthuis L (1980) FAO species catalogue. Shrimps and prawns of the world. An annotated catalogue of species of interest to fisheries FAO. Fish Synopsis 125:271
Jemil I, Abdelhedi O, Nasri R, Mora L, Jridi M, Aristoy MC, Toldrá F, Nasri M (2017) Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res Int 100:121–133
Jemil I, Jridi M, Nasri R, Ktari N, Ben Salem R, Mehiri M, Hajji M, Nasri M (2014) Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochem 49:963–972
Jemil I, Mora L, Nasri R, Abdelhedi O, Aristoy MC, Hajji M, Nasri M, Toldrá F (2016) A peptidomic approach for the identification of antioxidant and ACE-inhibitory peptides in sardinelle protein hydrolysates fermented by Bacillus subtilis A26 and Bacillus amyloliquefaciens An6. Food Res Int 89:347–358
Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ (2013) SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23:450–463
Jeuniaux C, Compère P, Goffinet G (1986) Structure, synthèse et dégradation des chitinoprotéines de la cuticule des crustacés décapodes. Ital J Zool 53:183–196
Kelainy EG, Laila IMI, Ibrahim SR (2019) The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ Sci Pollut Res 26:31675–31684
Kembhavi AA, Buttle DJ, Knight CG, Barrett AJ (1993) The two cysteine endopeptidases of legume seeds: purification and characterization by use of specific fluorometric assays. Arch Biochem Biophys 303:208–213
Khaled BH, Ktari N, Ghorbel Bellaaj O, Jridi M, Lassoued I, Nasri M (2014) Composition, functional properties and in vitro antioxidant activity of protein hydrolysates prepared from sardinelle (Sardinella aurita) muscle. J Food Sci Technol 51:622–633
Kirby AJ, Schmidt RJ (1997) The antioxidant activity of chinese herbs for eczema and of placebo herbs-I. J Ethnopharmacol 56:103–108
Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F (2013) Inhibition of angiotensin converting enzyme, human LDL cholesterol and DNA oxidation by hydrolysates from blacktip shark gelatin. J Food Sci Technol 51:177–182
Kumar A, Kumar D, George N, Sharma P, Gupta N (2018) A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of chitinase and chitin oligosaccharides. Int J Biol Macromol 109:263–272
Lee SJ, Kim KH, Kim YS, Kim EK, Hwang JW, Lim BO, Moon SH, Jeon BT, Jeon YJ, Ahn CB, Park PJ (2012) Biological activity from the gelatin hydrolysates of duck skin by-products. Process Biochem 47:1150–1154
Malomo SA, Aluko RE (2016) In vitro acetylcholinesterase-inhibitory properties of enzymatic hemp seed protein hydrolysates. J Am Oil Chem Soc 93:411–420
Maruthiah T, Somanath B, Immanuel G, Palavesam A (2015) Deproteinization potential and antioxidant property of haloalkalophilic organic solvent tolerant protease from marine Bacillus sp. APCMST-RS3 using marine shell wastes. Biotechnol Rep 8:124–132
Mechri S, Bouacem K, Amziane M, Dab A, Nateche F, Jaouadi B (2019a) Identification of a new serine alkaline peptidase from the moderately halophilic Virgibacillus natechei sp. nov., strain FarDT and its application as bioadditive for peptide synthesis and laundry detergent formulations. Biomed Res Int 2019:17
Mechri S, Bouacem K, Jabeur F, Mohamed S, Ammara Addou N, Dab A, Bouraoui A, Bouanane Darenfed A, Bejar S, Hacène H, Jaouadi B (2019b) Purification and biochemical characterization of a novel thermostable and halotolerant subtilisin SAPN, a serine protease from Melghiribacillus thermohalophilus Nari2AT for chitin extraction from crab and shrimp shell by-products. Extremophiles 23:529–547
Mechri S, Bouacem K, Zaraî Jaouadi N, Rekik H, Ben Elhoul M, Omrane Benmrad M, Hacéne H, Bejar S, Bouanane Darenfed A, Jaouadi B (2019c) Identification of a novel protease from the thermophilic bacterium Anoxybacillus kamchatkensis M1V. Extremophiles 23:687–709
Mhamdi S, Bkhairia I, Nasri R, Mechichi T, Nasri M, Kamoun AS (2017) Evaluation of the biotechnological potential of a novel purified protease BS1 from Bacillus safensis S406 on the chitin extraction and detergent formulation. Int J Biol Macromol 104:739–747
Muniyappan J, Varadharajan V, Namadevan P (2019) Biochemical screening and determination of bioactive components of commercially cultured pacific white shrimp Penaeus vannamei. Pharm Res 11:140–146
Nakchum L, Kim SM (2016) Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep Biochem Biotechnol 46:123–130
Oyaizu M (1988) Antioxidative activities of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. Jpn Soc Food Sci Technol 35:771–775
Prasasty V, Radifar M, Istyastono E (2018) Natural peptides in drug discovery targeting acetylcholinesterase. Molecules 23:2344
Pratt DE (1980) Natural antioxidants of soybeans and other oil-seeds. In: Simic MG, Karel M (eds) Autoxidation in food and biological systems. Springer, Boston, pp 283–293
Ramesh RR, Muralidharan V, Palanivel S (2018) Preparation and application of unhairing enzyme using solid wastes from the leather industry—an attempt toward internalization of solid wastes within the leather industry. Environ Sci Pollut Res 25:2121–2136
Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J (2007) Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol 45:328–336
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay free radical. Biol Med 26:1231–1237
Rebah FB, Miled N (2013) Fish processing wastes for microbial enzyme production: a review. 3 Biotech 3:255–265
Rødde RH, Einbu A, Vårum KM (2008) A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr Polym 71:388–393
Sachindra NM, Bhaskar N (2008) In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour Technol 99:9013–9016
Sahnoun M, Bejar S, Daoud L, Ayadi L, Brini F, Saibi W (2019) Effect of Agave americana L. on the human, and Aspergillus oryzae S2 alpha-amylase inhibitions. Nat Prod Res 33:755–758
Sayari N, Sila A, Abdelmalek BE, Abdallah RB, Ellouz-Chaabouni S, Bougatef A, Balti R (2016) Chitin and chitosan from the Norway lobster by-products: antimicrobial and anti-proliferative activities. Int J Biol Macromol 87:163–171
Schurink M, van Berkel WJ, Wichers HJ, Boeriu CG (2007) Novel peptides with tyrosinase inhibitory activity. Peptides 28:485–495
Seedevi P, Moovendhan M, Vairamani S, Shanmugam A (2017) Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int J Biol Macromol 99:519–529
Sellem I, Kaaniche F, Chakchouk AM, Mellouli L (2016) Anti-oxidant, antimicrobial and anti-acetylcholinesterase activities of organic extracts from aerial parts of three Tunisian plants and correlation with polyphenols and flavonoids contents. Bangladesh J Pharmacol 11:531–544
Sila A, Bougatef A (2016) Antioxidant peptides from marine by-products: isolation, identification and application in food systems. A review. J Funct Foods 21:10–26
Torino MI, Limón RI, Martínez Villaluenga C, Mäkinen S, Pihlanto A, Vidal Valverde C, Frias J (2013) Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem 136:1030–1037
Tu M, Wang C, Chen C, Zhang R, Liu H, Lu W, Jiang L, Du M (2018) Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem 256:98–104
Wang SL, Chang TJ, Liang TW (2010) Conversion and degradation of shellfish wastes by Serratia sp. TKU016 fermentation for the production of enzymes and bioactive materials. Biodegradation 21:321–333
Wang SL, Yeh PY (2006) Production of a surfactant-and solvent-stable alkaliphilic protease by bioconversion of shrimp shell wastes fermented by Bacillus subtilis TKU007. Process Biochem 41:1545–1552
Younes I, Ghorbel Bellaaj O, Nasri R, Chaabouni M, Rinaudo M, Nasri M (2012) Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem 47:2032–2039
Yu HC, Hsu JL, Chang CI, Tan FJ (2017) Antioxidant properties of porcine liver proteins hydrolyzed using Monascus purpureus. Food Sci Biotechnol 26:1217–1225
Yuan G, Li W, Pan Y, Wang C, Chen H (2018) Shrimp shell wastes: optimization of peptide hydrolysis and peptide inhibition of α-amylase. Food Biosci 25:52–60
Acknowledgments
The authors would like to thank Mrs. M. Mezghani Abid, and Mr. A. Hadj Brahim (LMBEB-CBS). Also, the authors were grateful to Mr. I. Hssairi, Mr. A. Zitoun, and Mr. F. Boukhili (Specialized unit, UVRR-CBS) for their technical assistance. Special thanks are also due Dr. Z. Bouallagui from the Centre of Biotechnology of Sfax (Sfax, Tunisia) and Pr. W. Hariz from the English Department at the Sfax Faculty of Sciences, University of Sfax, Tunisia, for constructive proofreading and language polishing services.
Funding
This study was supported by the Ministry of Higher Education and Scientific Research of Tunisia, under the Contract Program 2015-2019: grant no. LMBEE_CBS/code: LR15CBS06 and the Tunisian-Algerian project JAOUADI/BADIS_TNDZ-MicrooZymes_2012-2018/code: TA/04/2012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mechri, S., Sellem, I., Bouacem, K. et al. A biological clean processing approach for the valorization of speckled shrimp Metapenaeus monoceros by-product as a source of bioactive compounds. Environ Sci Pollut Res 27, 15842–15855 (2020). https://doi.org/10.1007/s11356-020-08076-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08076-w