Abstract
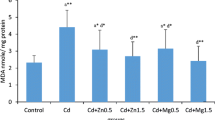
The present study was conducted to examine the nephrotoxic effects of heavy metals including lead (Pb), manganese (Mn), arsenic (As), and cadmium (Cd) in diabetic and non-diabetic Wistar rats. Animals were exposed to heavy metals for 30 days, Pb was injected as lead acetate (C4H6O4Pb), Mn was injected as manganese chloride (MnCl2), Cd was injected as cadmium chloride (CdCl2), and As was administered orally to rats in the form of sodium arsenite (AsO2Na). Results showed that metal deposition trends in tissues were Pb > As > Cd > Mn and the urinary metal levels were Pb > Cd > As > Mn. Diabetic metal alone, as well as metal mixture-treated groups, showed decreased urinary metal levels as compared with non-diabetic metal alone and metal mixture-treated groups. Both diabetic- and non-diabetic metal mixture-treated groups revealed an increasing trend of blood urea nitrogen (BUN) and serum creatinine. In addition, heavy metal treatments resulted in elevated malondialdehyde (MDA) levels in the kidney tissue while decreased levels of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GHS) were observed in the kidney tissue in comparison with the control group. The histological analysis of the kidney tissues showed tubular degeneration, fibrosis, and vacuolation as a result of heavy metal exposure. The present study revealed that co-exposure of heavy metals (Pb, Cd, Mn, As) induced more nephrotoxicity as compared with the metal alone treatment. Moreover, diabetic Wistar rats are more prone to kidney damage as a result of heavy metal exposure.





Similar content being viewed by others
References
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int J Environ Res Public Health 16(2):274
Andrade V, Mateus ML, Batoréu MC, Aschner M, dos Santos AM (2013) Urinary delta-ALA: a potential biomarker of exposure and neurotoxic effect in rats co-treated with a mixture of lead, arsenic and manganese. Neurotoxicology 38:33–41
Andrade V, Mateus ML, Santos D, Aschner M, Batoreu MC, Dos Santos AM (2014) Arsenic and manganese alter lead deposition in the rat. Biol Trace Elem Res 158:384–391
Badran M, Morsy R, Soliman H, Elnimr T (2016) Assessment of trace elements levels in patients with type 2 diabetes using multivariate statistical analysis. J Trace Elem Med Biol 33:114–119
Baş H, Kalender Y (2015) Nephrotoxic effects of lead nitrate exposure in diabetic and nondiabetic rats: involvement of oxidative stress and the protective role of sodium selenite. Environ Toxicol 31:1229–1240
Chandramohan R, Saravanan S, Pari L (2017) Beneficial effects of tyrosol on altered glycoprotein components in streptozotocin-induced diabetic rats. Pharm Biol 55:1631–1637
Chen L, Jin T, Huang B, Nordberg G, Nordberg M (2006) Critical exposure level of cadmium for elevated urinary metallothionein—an occupational population study in China. Toxicol Appl Pharmacol 215(1):93–99
Cobbina SJ, Chen Y, Zhou Z, Wu X, Zhao T, Zhang Z, Yang L (2015) Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J Hazard Mater 294:109–120
Dutta RP, Patil MB (2018) Therapeutic potential of root and stem bark of wild medicinal plant Ziziphusmauritiana (Lamk.) against silica induced toxicity in Wistar albino rats. J Ethnopharmacol 224:111–118
Garçon G, Leleu B, Marez T, Zerimech F, Haguenoer JM, Furon D, Shirali P (2007) Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: usefulness of alpha-glutathione S-transferase. Sci Total Environ 377(2-3):165–172
Gawlik M, Gawlik MB, Smaga I, Filip M (2017) Manganese neurotoxicity and protective effects of resveratrol and quercetin in preclinical research. Pharmacol Rep 69:322–330
Gunawardana CG, Martinez RE, Xiao W, Templeton DM (2006) Cadmium inhibits both intrinsic and extrinsic apoptotic pathways in renal mesangial cells. Am J Physiol Ren Physiol 290(5):F1074–F1082
Hambach R, Lison D, D’haese PC, Weyler J, De Graef E, De Schryver A, ..., Van Sprundel M (2013) Co-exposure to lead increases the renal response to low levels of cadmium in metallurgy workers. Toxicol Lett, 222(2):233–238
Hamed EA, Meki ARM, El-Mottaleb NAA (2010) Protective effect of green tea on lead-induced oxidative damage in rat’s blood and brain tissue homogenates. J Physiol Biochem 66(2):143–151
Han SG, Castranova V, Vallyathan V (2007) Comparative cytotoxicity of cadmium and mercury in a human bronchial epithelial cell line (BEAS-2B) and its role in oxidative stress and induction of heat shock protein 70. J Toxic Environ Health A 70(10):852–860
Hanafy S, Soltan ME (2004) Effects of vitamin E pretreatment on subacute toxicity of mixture of Co, Pb, and Hg nitrate-induced nephrotoxicity in rats. Environ Toxicol Pharmacol 17(3):159–167
Haridy M, Al-Amgad Z, Sakai H, Mohi-Eldin M (2014) Ameliorating effects of garlic, calcium, and vitamin C on chronic lead toxicity in albino rats. Comp Clin Pathol 23:1215–1223
Huang M, Choi SJ, Kim DW, Kim NY, Park CH, Yu SD, Choi BS (2009) Risk assessment of low-level cadmium and arsenic on the kidney. J Toxic Environ Health A 72(21-22):1493–1498
Jadhav SH, Sarkar SN, Patil RD, Tripathi HC (2007) Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: a biochemical and histopathological study in male rats. Arch Environ Contam Toxicol 53(4):667–677
Jamakala O, Rani UA (2015) Amelioration effect of zinc and iron supplementation on selected oxidative stress enzymes in liver and kidney of cadmium-treated male albino rat. Toxicol Int 22(1):1
Jia Q, Ha X, Yang Z, Hui L, Yang X (2012) Oxidative stress: a possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol Mech Methods 22(9):705–710
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23(5):783–792
Kasperczyk S, Dobrakowski M, Kasperczyk J, Ostałowska A, Zalejska-Fiolka J, Birkner E (2014) Beta-carotene reduces oxidative stress, improves glutathione metabolism and modifies antioxidant defense systems in lead-exposed workers. Toxicol Appl Pharmacol 280(1):36–41
Khan AR, Awan FR (2014) Metals in the pathogenesis of type 2 diabetes. J Diabetes Metab Disord 13(1):16
Król E, Bogdański P, Suliburska J, Krejpcio Z (2018) The relationship between dietary, serum and hair levels of minerals (Fe, Zn, Cu) and glucose metabolism indices in obese type 2 diabetic patients. Biol Trace Elem Res:1–11
Lu SC (1999) Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13(10):1169–1183
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenientassay for superoxide dismutase. Eur J Biochem 47(3):469–474
Matović V, Buha A, Ðukić-Ćosić D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140
Moneim AEA, Dkhil MA, Al-Quraishy S (2011) The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater 194:250–255
Nair A, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 14(3):6116–6143
Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V (2009) Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 170(9):1156–1164
Noeman SA, Hamooda HE, Baalash AA (2011) Biochemical study of oxidative stress markers in the liver, kidney and heart of high fatdiet induced obesity in rats. Diabetol Metab Syndr 3(1):17
Nordberg GF, Jin T, Hong F, Zhang A, Buchet JP, Bernard A (2005) Biomarkers of cadmium and arsenic interactions. Toxicol Appl Pharmacol 206(2):191–197
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24(1):66–73
Rahman A, Yamazaki D, Sufiun A, Kitada K, Hitomi H, Nakano D, Nishiyama A (2018) A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS One 13(2):e0192531
Renuka M, Suneetha Y, Srinivasulu RM (2017) Cadmium induced oxidative stress in wistar rats: ameliorative effect of quercetin and embilica officinalis plant extracts. Toxicol Forensic Med Open J 2(1):26–35
Riant M, Meirhaeghe A, Giovannelli J, Occelli F, Havet A, Cuny D, Amouyel P, Dauchet L (2018) Associations between long-term exposure to air pollution, glycosylated hemoglobin, fasting blood glucose and diabetes mellitus in northern France. Environ Int 120:121–129
Roggenbeck BA, Banerjee M, Leslie EM (2016) Cellular arsenic transport pathways in mammals. J Environ Sci 49:38–58
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Soliman MM, Baiomy AA, Yassin MH (2015) Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in Wistar rats. Biol Trace Elem Res 167(1):91–102
Sudjarwo SA, Eraiko K, Sudjarwo GW, Koerniasari (2017) Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran J Basic Med Sci 20(11):1227–1231
Thijssen S, Cuypers A, Maringwa J, Smeets K, Horemans N, Lambrichts I, Van Kerkhove E (2007) Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 236(1-2):29–41
Tsai TL, Kuo CC, Pan WH, Chung YT, Chen CY, Wu TN, Wang SL (2017) The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int 92(3):710–720
Turner T, Chen X, Zahner M, Opsahl A, DeMarco G, Boucher M, Perreault M (2018) FGF21 increases water intake, urine output and blood pressure in rats. PLoS One 13(8):e0202182
Wang G, Fowler BA (2008) Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol Appl Pharmacol 233(1):92–99
Wang JP, Wang SL, Lin Q, Zhang L, Huang D, Ng JC (2009) Association of arsenic and kidney dysfunction in people with diabetes and validation of its effects in rats. Environ Int 35(3):507–511
Wei H, Meng Z (2011) Protective effects of epigallocatechin-3-gallate against lead-induced oxidative damage. Human & Experimental Toxicology 30(10):1521-1528
Winiarska-Mieczan A (2015) The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure–a rat model study. Regul Toxicol Pharmacol 73(2):521–529
Wolide AD, Zawdie B, Alemayehu T, Tadesse S (2016) Evaluation of serum ferritin and some metal elements in type 2 diabetes mellitus patients: comparative cross-sectional study. Diabetes Metab Syndr Obes 9:417–424
Xing Y, Xia W, Zhang B, Zhou A, Huang Z, Zhang H, ... Xu S (2018) Relation between cadmium exposure and gestational diabetes mellitus. Environ Int 113:300–305
Yabe J, Nakayama SM, Ikenaka Y, Muzandu K, Ishizuka M, Umemura T (2011) Uptake of lead, cadmium, and other metals in the liver and kidneys of cattle near a lead-zinc mine in Kabwe, Zambia. Environ Toxicol Chem 30(8):1892–1897
Yu J, Fujishiro H, Miyataka H, Oyama TM, Hasegawa T, Seko Y, Himeno S (2009) Dichotomous effects of lead acetate on the expression of metallothionein in the liver and kidney of mice. Biol Pharm Bull 32(6):1037–1042
Yurekli M, Esrefoglu M, IlkerDoğru M, Doğru A, Gul M, Whidden M (2009) Adrenomedullin reduces antioxidant defense system and enhances kidney tissue damage in cadmium and lead exposed rats. Environ Toxicol 24(3):279–286
Zhang S, Jin Y, Zeng Z, Liu Z, Fu Z (2015) Subchronic exposure of mice to cadmium perturbs their hepatic energy metabolism and gut microbiome. Chem Res Toxicol 28(10):2000–2009
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riaz, M.A., Nisa, Z.U., Mehmood, A. et al. Metal-induced nephrotoxicity to diabetic and non-diabetic Wistar rats. Environ Sci Pollut Res 26, 31111–31118 (2019). https://doi.org/10.1007/s11356-019-06022-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06022-z