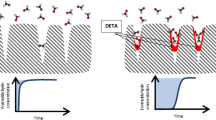
Abstract
Amine-modified diatomite with remarkable formaldehyde (HCHO) removal efficiency was prepared by grafting 3-aminopropyltrimethoxysilane (APTMS) in this research. The interfacial properties and microstructures of the prepared adsorbents were characterized and analyzed. The HCHO adsorption properties of the amine modified diatomite were also systematically studied, and it has been proven to be effective adsorbent with better adsorption performance than activated carbon for the removal of gaseous HCHO. Furthermore, to better explain the experimental results, we performed density functional theory (DFT) study on the adsorption system and calculated the geometry, energy, and charge parameters based on first principles. Also, the underlying adsorption mechanism was proposed detailedly by combining experimentation with DFT calculation, suggesting that amine modified diatomite can be efficient adsorbent for the elimination of gaseous formaldehyde.










Similar content being viewed by others

References
Acres RG, Ellis AV, Alvino J, Lenahan CE, Khodakov DA, Metha GF, Andersson GG (2012) Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J Phys Chem C 116:6289–6297
Aivalioti M, Vamvasakis I, Gidarakos E (2010) BTEX and MTBE adsorption onto raw and thermally modified diatomite. J Hazard Mater 178:136–143
Araghi SH, Entezari MH, Chamsaz M (2015a) Modification of mesoporous silica magnetite nanoparticles by 3-aminopropyltriethoxysilane for the removal of Cr (VI) from aqueous solution. Microporous Mesoporous Mater 218:101–111
Araghi SH, Entezari MH, Googheri MSS (2015b) Configurational study of amino-functionalized silica surfaces: a density functional theory modeling. J Mol Graph Modell 59:21–30
Bernabe DP, Herrera RAS, Doma B, Fu M-L, Dong Y, Wang Y-F (2015) Adsorption of low concentration formaldehyde in air using ethylene-diamine-modified diatomaceous earth. Aerosol Air Qual Res 15:1652–1661
Chen N, Zhang Z, Feng C, Zhu D, Yang Y, Sugiura N (2011) Preparation and characterization of porous granular ceramic containing dispersed aluminum and iron oxides as adsorbents for fluoride removal from aqueous solution. J Hazard Mater 186:863–868
Chen X, Xu L, Liu L-L, Zhao L-S, Chen C-P, Zhang Y, Wang X-C (2017) Adsorption of formaldehyde molecule on the pristine and transition metal doped graphene: first-principles study. Appl Surf Sci 396:1020–1025
Chi C, Chen W, Guo M, Weng M, Yan G, Shen X (2016) Law and features of TVOC and formaldehyde pollution in urban indoor air. Atmos Environ 132:85–90
Cottet L, Almeida C, Naidek N, Viante M, Lopes M, Debacher N (2014) Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Appl Clay Sci 95:25–31
de Luna MDG, Laciste MT, Tolosa NC, Lu M-C (2018) Effect of catalyst calcination temperature in the visible light photocatalytic oxidation of gaseous formaldehyde by multi-element doped titanium dioxide. Environ Sci Pollut Res 25:15216–15225
El-Shahawi M, Bashammakh A, Alwael H, Alsibaai A, Dowaidar A (2017) Adsorption characteristics of polycyclic aromatic hydrocarbons from non-aqueous media using activated carbon derived from phenol formaldehyde resin: kinetics and thermodynamic study. Environ Sci Pollut Res 24:4228–4240
Ewlad-Ahmed AM, Morris MA, Patwardhan SV, Gibson LT (2012) Removal of formaldehyde from air using functionalized silica supports. Environ Sci Technol 46:13354–13360
Fan J, Gou X, Sun Y, Ran X, Teng W, Wang X (2017) Adsorptive performance of chromium-containing ordered mesoporous silica on volatile organic compounds (VOCs). Nat Gas Ind B 4:382–389
Haselbach LM, Ma S (2008) Potential for carbon adsorption on concrete: surface XPS analyses. Environ Sci Technol 42:5329–5344
Hu S-C, Chen Y-C, Lin X-Z, Shiue A, Huang P-H, Chen Y-C, Chang S-M, Tseng C-H, Zhou B (2018) Characterization and adsorption capacity of potassium permanganate used to modify activated carbon filter media for indoor formaldehyde removal. Environ Sci Pollut Res 25:28525–28545
Hu Z, Zheng S, Jia M, Dong X, Sun Z (2017) Preparation and characterization of novel diatomite/ground calcium carbonate composite humidity control material. Adv Powder Technol 28:1372–1381
Idris SA, Davidson CM, Mcmanamon C, Morris MA, Anderson P, Gibson LT (2011) Large pore diameter MCM-41 and its application for lead removal from aqueous media. J Hazard Mater 185:898–904
Kim DI, Park JH, Kim SD, Lee JY, Yim JH, Jeon JK, Park SH, Park YK (2011) Comparison of removal ability of indoor formaldehyde over different materials functionalized with various amine groups. J Ind Eng Chem 17:1–5
Lashaki MJ, Atkinson JD, Hashisho Z, Phillips JH, Anderson JE, Nichols M, Misovski T (2016) Effect of desorption purge gas oxygen impurity on irreversible adsorption of organic vapors. Carbon 99:310–317
Le Y, Guo D, Cheng B, Yu J (2013) Bio-template-assisted synthesis of hierarchically hollow SiO2 microtubes and their enhanced formaldehyde adsorption performance. Appl Surf Sci 274:110–116
Li C, Sun Z, Ma R, Xue Y, Zheng S (2017) Fluorine doped anatase TiO2 with exposed reactive (001) facets supported on porous diatomite for enhanced visible-light photocatalytic activity. Microporous Mesoporous Mater 243:281–290
Li C, Sun Z, Song A, Dong X, Zheng S, Dionysiou DD (2018) Flowing nitrogen atmosphere induced rich oxygen vacancies overspread the surface of TiO2/kaolinite composite for enhanced photocatalytic activity within broad radiation spectrum. Appl Catal B 236:76–87
Lin F, Zhu G, Shen Y, Zhang Z, Dong B (2015) Study on the modified montmorillonite for adsorbing formaldehyde. Appl Surf Sci 356:150–156
Liu Y, Jia H, Sun Z, Pan Y, Zhang G, Zheng S (2019) High-efficiency removal of gaseous HCHO by amine functionalized natural opoka. Chem Phys Lett 722C:32–38
Luengas A, Barona A, Hort C, Gallastegui G, Platel V, Elias A (2015) A review of indoor air treatment technologies. Rev EnvironSci Biotechnol 14:499–522
Ma D, Ju W, Li T, Yang G, He C, Ma B, Tang Y, Lu Z, Yang Z (2016) Formaldehyde molecule adsorption on the doped monolayer MoS2: A first-principles study. Appl Surf Sci 371:180–188
Noorizadeh S, Shakerzadeh E (2012) Formaldehyde adsorption on pristine, Al-doped and mono-vacancy defected boron nitride nanosheets: a first principles study. Comput Mater Sci 56:122–130
Ochs SM, Grotz LO, Factorine LS, Rodrigues MR, Netto ADP (2012) Occupational exposure to formaldehyde in an institute of morphology in Brazil: a comparison of area and personal sampling. Environ Sci Pollut Res 19:2813–2819
Palimi MJ, Rostami M, Mahdavian M, Ramezanzadeh B (2014) Surface modification of Fe2O3 nanoparticles with 3-aminopropyltrimethoxysilane (APTMS): An attempt to investigate surface treatment on surface chemistry and mechanical properties of polyurethane/Fe2O3 nanocomposites. Appl Surf Sci 320:60–72
Qiao B, Wang T-J, Gao H, Jin Y (2015) High density silanization of nano-silica particles using γ-aminopropyltriethoxysilane (APTES). Appl Surf Sci 351:646–654
Ren Z, Gao H, Zhang H, Liu X (2014) Effects of fluxes on the structure and filtration properties of diatomite filter aids. Int J Miner Process 130:28–33
Salman M, Athar M, Shafique U, Rehman R, Ameer S, Ali SZ, Azeem M (2012) Removal of formaldehyde from aqueous solution by adsorption on kaolin and bentonite: a comparative study. Turk J Eng Environ Sci 36:263–270
Sun Z, Li C, Yao G, Zheng S (2016) In situ generated g-C3N4/TiO2 hybrid over diatomite supports for enhanced photodegradation of dye pollutants. Mater Des 94:403–409
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069
Vilarrasa-García E, Cecilia JA, Azevedo DCS, Jr CLC, Rodríguez-Castellón E (2017a) Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture. Microporous Mesoporous Mater 249:25–33
Vilarrasa-García E, Cecilia JA, Bastos-Neto M, Jr CLC, Azevedo DCS, Rodríguez-Castellón E (2017b) Microwave-assisted nitric acid treatment of sepiolite and functionalization with polyethylenimine applied to CO2 capture and CO2/N2 separation. Appl Surf Sci 410:315–325
Wang G, Miao Y, Sun Z, Zheng S (2018) Simultaneous adsorption of aflatoxin B1 and zearalenone by mono-and di-alkyl cationic surfactants modified montmorillonites. J Colloid Interface Sci 511:67–76
Wang Z, Zhong M, Chen L (2016) Coal-based granular activated carbon loaded with MnO2 as an efficient adsorbent for removing formaldehyde from aqueous solution. Desalin Water Treat 57:13225–13235
Wen Q, Li C, Cai Z, Zhang W, Gao H, Chen L, Zeng G, Shu X, Zhao Y (2011) Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde. Bioresour Technol 102:942–947
Xu Z, Yu J, Low J, Jaroniec M (2014) Microemulsion-assisted synthesis of mesoporous aluminum oxyhydroxide nanoflakes for efficient removal of gaseous formaldehyde. ACS Appl Mater Interfaces 6:2111–2117
Yang S, Zhu Z, Wei F, Yang X, Yang S, Zhu Z, Wei F, Yang X (2017) Enhancement of formaldehyde removal by activated carbon fiber via in situ growth of carbon nanotubes. Build Environ 126:27–33
Ye J, Zhu X, Cheng B, Yu J, Jiang C (2016) Few-layered graphene-like boron nitride: a highly efficient adsorbent for indoor formaldehyde removal. Environ Sci Technol Lett 4:20–25
Yi Y, Li C, Zhao L, Du X, Gao L, Chen J, Zhai Y, Zeng G (2018) The synthetic evaluation of CuO-MnOx-modified pinecone biochar for simultaneous removal formaldehyde and elemental mercury from simulated flue gas. Environ Sci Pollut Res 25:4761–4775
Zaitan H, Korrir A, Chafik T, Bianchi D (2013) Evaluation of the potential of volatile organic compound (di-methyl benzene) removal using adsorption on natural minerals compared to commercial oxides. J Hazard Mater 262:365–376
Zendehdel R, Vahabi M, Sedghi R (2018) Estimation of formaldehyde occupational exposure limit based on genetic damage in some Iranian exposed workers using benchmark dose method. Environ Sci Pollut Res 25:31183–31189
Zhang D, Wu J, Peng L, Cao Y, Zhang D, Wu J, Peng L, Cao Y (2017a) Room-temperature SO2 gas sensing properties based on metal-doped MoS2 nanoflower: an experimental and density functional theory Investigation. J Mater Chem A 5:20666–20667
Zhang G, Sun Z, Duan Y, Ma R, Zheng S (2017b) Synthesis of nano-TiO2 /diatomite composite and its photocatalytic degradation of gaseous formaldehyde. Appl Surf Sci 412:105–112
Zhang G, Liu Y, Zheng S, Hashisho Z (2019) Adsorption of volatile organic compounds onto natural porous minerals. J Hazard Mater 364:317–324
Zhang S, Chen H, Wang A, Liu Y, Hou H, Hu Q (2018) Combined effects of co-exposure to formaldehyde and acrolein mixtures on cytotoxicity and genotoxicity in vitro. Environ Sci Pollut Res 25:25306–25314
Zhou Q, Yuan L, Yang X, Fu Z, Tang Y, Wang C, Zhang H (2014) DFT study of formaldehyde adsorption on vacancy defected graphene doped with B, N, and S. Chem Phys 440:80–86
Zhou Q, Ju W, Su X, Yong Y, Li X (2017) Adsorption behavior of SO2 on vacancy-defected graphene: A DFT study. J Phys Chem Solids 109:40–45
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Key R&D Program of China (2017YFB0310803), the Key R&D Program of Jilin (20180201078GX), the Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), the Yue Qi Young Scholar Project, China university of Mining &Technology (Beijing) and the Fundamental Research Funds for the Central Universities (2010YH10 and 2015QH01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 384 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Jia, H., Li, C. et al. Efficient removal of gaseous formaldehyde by amine-modified diatomite: a combined experimental and density functional theory study. Environ Sci Pollut Res 26, 25130–25141 (2019). https://doi.org/10.1007/s11356-019-05758-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05758-y