Abstract
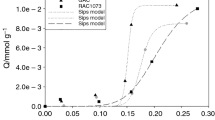
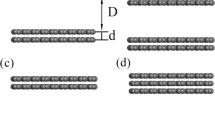
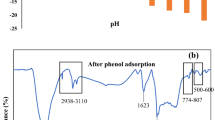
Modification of the surface of raw activated carbon using chemical solvents can significantly improve the adsorption performance of activated carbon. Triethylenetetramine is one of the most important chemical solvents used to modify raw activated carbon for formaldehyde removal indoor. We conducted the liquid impregnation experiments at different initial concentrations, temperatures, adsorbent dosage and time ranges to fully investigate the adsorption of triethylenetetramine on the surface of raw activated carbon for modification. We found that the Langmuir isotherm model and pseudo-first-order kinetic model fit quite well with the experimental data and the R2 are 0.9883 and 0.9954, respectively. The theoretical maximum adsorption capacity is 166.67 mg/g. The change in Gibbs free energy (ΔG0), enthalpy change (ΔH0) and entropy change (ΔS0) were also calculated to study the direction and driving force of the liquid adsorption process. In order to understand the adsorption process at the molecular level, a new activated carbon model based on the actual physical and chemical properties of activated carbon was carefully established in the Materials Studio to simulate the liquid-phase adsorption. The pore structure, elemental composition, functional group content, density, pore volume, and porosity of the activated carbon model converge close to the actual activated carbon and the adsorption isotherms obtained from the simulation agree well with the experimental results. The results show that the adsorption of triethylenetetramine on activated carbon is a spontaneous, endothermic and monolayer physical adsorption process.













Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Laskar II, Hashisho Z, Phillips JH, Anderson JE, Nichols M (2019) Modeling the Effect of Relative Humidity on Adsorption Dynamics of Volatile Organic Compound onto Activated Carbon. Environ Sci Technol 53:2647–2659
Latifatu M, Park JH, Ko JM, Park J (2018) Supercapacitive properties of composite electrode consisting of activated carbon and quinone derivatives. J Ind Eng Chem 63:12–18
Chang SM, Hu SC, Shiue A, Lee PY, Leggett G (2020) Adsorption of silver nano-particles modified activated carbon filter media for indoor formaldehyde removal. Chem Phys Lett 757:137864
Nazzal JS, Kiełbasa K (2019) Advances in modification of commercial activated carbon for enhancement of CO2 capture. Appl Surf Sci 494:137–151
Giraldo L, Vargas DP, Moreno-Piraján JC (2020) Study of CO2 Adsorption on Chemically Modified Activated Carbon with Nitric Acid and Ammonium Aqueous. Front Chem 8:543452
Wang J, Adelodun AA, Oh JM, Jo YM (2020) TEPA impregnation of electrospun carbon nanofibers for enhanced low-level CO2 adsorption. Nano Converg 7(1):1–11
Wang J, Wu Z, Niu Q, Liu L, Yang L, Fu M, Ye D, Chen P (2021) Highly efficient adsorptive removal of toluene using silicon-modified activated carbon with improved fire resistance. J Hazard Mater 415:125753
Li L, Liu S, Liu J (2011) Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J Hazard Mater 192(2):683–690
Natheer RT, Saleh MY (2021) Removal of Pb (II) ions from Tigris river wastewater in Mosul city by using modified commercial activated carbon. Egypt J Chem 64:2–3
Jin G, Eom Y, Lee TG (2016) Removal of Hg (II) from aquatic environments using activated carbon impregnated with humic acid. J Ind Eng Chem 42:46–52
Kazeem TS, Lateef SA, Ganiyu SA, Qamaruddin M, Tanimu A, Sulaiman KO, Jillani SM, Alhooshani K (2018) Aluminium-modified activated carbon as efficient adsorbent for cleaning of cationic dye in wastewater. J Clean Prod 205:303–312
Ahmad W, Amin A, Rehman TU, Hussain F, Ilyas M (2022) Treatment of dyes contaminated water using surfactants modified activated carbon derived from rice husk. Desalin Water Treat 248:288–299
Yang Z, Zhao Z, Yang X, Ren Z (2021) Xanthate modified magnetic activated carbon for efficient removal of cationic dyes and tetracycline hydrochloride from aqueous solutions. Colloid Surf A-Physicochem Eng Asp 615:126273
Sahu N, Singh J, Koduru JR (2021) Removal of arsenic from aqueous solution by novel iron and iron–zirconium modified activated carbon derived from chemical carbonization of Tectona grandis sawdust: Isotherm, kinetic, thermodynamic and breakthrough curve modelling. Environ Res 200:111431
Tian C, Feng C, Wei M, Wu Y (2018) Enhanced adsorption of anionic toxic contaminant Congo Red by activated carbon with electropositive amine modification. Chemosphere 208:476–483
Eskandari P, Farhadian M, Solaimany Nazar AR, Goshadrou A (2021) Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: a comparative study. Int J Environ Sci Technol 18:297–316
Baur GB, Spring J, Kiwi-Minsker L (2018) Amine functionalized activated carbon fibers as effective structured adsorbents for formaldehyde removal. Adsorpt J Int Adsorpt Soc 24:725–732
Xu GM, Chen B, Guo B, He DX, Yao SZ (2011) Detection of intermediates for the Eschweiler-Clarke reaction by liquid-phase reactive desorption electrospray ionization mass spectrometry. Analyst 136:2385–2390
Zhao WB, Liu B, Chen J (2014) Preparation of amino-modified pan fibers with triethylenetetramine as aminating reagents and their application in CO2 adsorption. J Nanomater 13:24–33
Ge HC, Ma ZW (2015) Microwave preparation of triethylenetetramine modified graphene oxide/chitosan composite for adsorption of Cr (VI). Carbohydr Polym 131:280–287
Ngamkitpinyo N, Imyim A (2015) Selective extraction of Pb (II) using triethylenetetramine-modified polystyrene-divinylbenzene resin. J Mater Environ Sci 6:1562–1569
Li ZH, Chang PH, Jiang WT (2019) Mechanisms of Cu2+, triethylenetetramine (TETA), and Cu-TETA sorption on rectorite and its use for metal removal via metal-TETA complexation. J Hazard Mater 373:187–196
Huang Y, Cheng QF, Wang Z, Liu SY, Zou CW, Guo JS, Guo XJ (2020) Competitive adsorption of benzene and water vapor on lignite-based activated carbon: Experiment and molecular simulation study. Chem Eng J 398:125557
An YX, Fu Q, Zhang DH, Wang YY, Tang ZL (2019) Performance evaluation of activated carbon with different pore sizes and functional groups for VOC adsorption by molecular simulation. Chemosphere 227:9–16
Kowalczyk P, Miyawaki J, Azuma Y, Yoon SH, Nakabayashi K, Gauden PA, Furmaniak S, Terzyk AP, Wisniewski M, Wloch J, Kaneko K, Neimark AV (2017) Molecular simulation aided nanoporous carbon design for highly efficient low-concentrated formaldehyde capture. Carbon 124:152–160
Kohmuean P, Inthomya W, Wongkoblap A, Tangsathitkulchai C (2021) Monte Carlo Simulation and Experimental Studies of CO2, CH4 and Their Mixture Capture in Porous Carbons. Molecules 26:2413
Segarra EI, Glandt ED (1994) Model microporous carbons: microstructure, surface polarity and gas adsorption. Chem Eng Sci 49:2953–2965
Li S, Song KL, Zhao DF, Rugarabamu JR, Diao R, Gu YY (2020) Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Microporous Mesoporous Mat 302:110220
Jaramillo J, Álvarez PM, Gómez-Serrano V (2010) Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl Surf Sci 256:5232–5236
Fan X, Parker DJ, Smith MD (2003) Preparation and ozone-surface modification of activated carbon Thermal stability of oxygen surface groups. Water Res 37:4929–4937
Kilic M, Varol EA, Pütün AE (2011) Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: Equilibrium, kinetics and thermodynamics. J Hazard Mater 189:397–403
Freundlich H (1907) Über die Adsorption in Lösungen. Z Phys Chem 57:385–470
Dubinin MM, Radushkevich LV (1947) Proc Acad Sci USSR. Phys Chem Sect 55:331
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J Hazard Mater 162:616–645
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ho YS, McKay G, Wase DAJ, Foster CF (2000) Study of the Sorption of Divalent Metal Ions on to Peat, Adsorp. Sci Technol 18:639–650
Weber WJ, Morriss JC (1963) Kinetics of Adsorption on Carbon from Solutions. J Sanit Eng Div Am Soc Civil Eng 89:31–60
Mathews JP, Chaffee AL (2012) The molecular representations of coal – A review. Fuel 96:1–14
Tang XD, Ran G, Li JJ, Zhang ZQ, Xiang CX (2021) Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J Hazard Mater 402:123579
Liu BT, Younis SA, Lee J, Szulejko JE, Dou XM, Kim KH (2021) The competing role of moisture in adsorption of gaseous benzene on microporous carbon. Sep Purif Technol 277:119487
Sedaghat S, Ahadian MM, Jafarian M, Hatamie S (2019) Model Fuel Deep Desulfurization Using Modified 3D Graphenic Adsorbents: Isotherm, Kinetic, and Thermodynamic Study. Ind Eng Chem Res 58:10341–10351
Na CJ, Yoo MJ, Tsang DCW, Kim HW, Kim KH (2019) High-performance materials for effective sorptive removal of formaldehyde in air. J Hazard Mater 366:452–465
Liu L, Tan SJ, Horikawa T, Do DD, Liu JJ (2017) Water adsorption on carbon - A review. Adv Colloid Interface Sci 250:64–78
Gu ZK, Zhu CY, Huang ZQ, Xu MH, Gong L (2021) Numerical simulations on the adsorption characteristics of aromatics on activated carbon by the GCMC method. Case Stud Therm Eng 26:101116
Degs YS, Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment 77:16–23
Tsai JH, Chiang HM, Huang GY, Chiang HL (2008) Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granular activated carbon and activated carbon fibers. J Hazard Mater 154:1183–1191
Recepoglu YK, Goren AY, Orooji Y, Khataee A (2022) Carbonaceous materials for removal and recovery of phosphate species: Limitations, successes and future improvement. Chemosphere 2:287
Tran HN, Tomul F, Ha NTH, Nguyen DT, Lima EC, Le GT, Chang CT, Masindi V, Woo SH (2020) Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J Hazard Mater 394:122255
Chen L, Zuo L, Jiang ZX, Jiang S, Liu KY, Tan JQ, Zhang LC (2019) Mechanisms of shale gas adsorption: Evidence from thermodynamics and kinetics study of methane adsorption on shale. Chem Eng J 361:559–570
Acknowledgements
This research is supported by the National Natural Science Foundation of China (No. 21978330).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Ma, X.C., He, C. et al. Experiment and molecular simulation for liquid phase adsorption of triethylenetetramine on activated carbon: equilibrium, kinetics, thermodynamics and molecular behavior. Carbon Lett. 33, 1977–1991 (2023). https://doi.org/10.1007/s42823-023-00589-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00589-x