Abstract
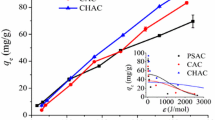
Sonicated activated carbon (SAC) was developed and used to remove ibuprofen and ketoprofen from aqueous media by adsorption. A standard activated carbon sample (AC) was used as comparison. Both adsorbents were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), N2 adsorption isotherms (Brunauer, Emmett, and Teller (BET)), helium gas pycnometry, and scanning electron microscopy (SEM). In the adsorption study, kinetics, equilibrium, and thermodynamics were evaluated. SAC presented better characteristics than AC. Pseudo-second-order model was adequate to predict the kinetic curves. The isotherm data obeyed the Sips model. Thermodynamic results revealed a spontaneous and endothermic process, where physisorption was involved. The maximum adsorption capacities of SAC were 134.5 and 89.2 mg g−1 for ibuprofen and ketoprofen, respectively. For AC, the maximum adsorption capacities were 115.1 and 79.1 mg g−1 for ibuprofen and ketoprofen, respectively. The sonication technique presented great potential to improve the AC characteristics, generating a promising material (SAC) for ibuprofen and ketoprofen adsorption.











Similar content being viewed by others
Abbreviations
- V P :
-
Pore volume, cm3 g−1
- R% :
-
Pharmaceutical removal percentage, %
- C 0 :
-
Initial pharmaceutical concentration, mg L−1
- C e :
-
Equilibrium pharmaceutical concentration, mg L−1
- q e :
-
Adsorption capacity at equilibrium, mg g−1
- q t :
-
Adsorption capacity at any time, mg g−1
- m :
-
Amount of adsorbent, g
- V :
-
Volume of solution, L
- q 1 :
-
Theoretical value for the adsorption capacity of PFO model, mg g−1
- k 1 :
-
Rate constant of PFO model, min−1
- q 2 :
-
Theoretical value for the adsorption capacity of PSO model, mg g−1
- k 2 :
-
Rate constant of PSO model, g mg−1 min−1
- t :
-
Time, min
- k F :
-
Freundlich constant, (mg g−1)(mg L−1)–1/nF
- 1/n F :
-
Heterogeneity factor, dimensionless
- q m :
-
Maximum adsorption capacity, mg g−1
- k L :
-
Langmuir constant, L mg−1
- k S :
-
Sips constant, L mg−1
- m :
-
Sips isotherm model exponent
- R 2 :
-
Coefficient of determination, dimensionless
- R 2 adj :
-
Adjusted coefficient of determination, dimensionless
- ARE :
-
Average relative error, %
- AIC :
-
Akaike information criterion, dimensionless
- ΔG o :
-
Standard Gibbs free energy change, kJ mol−1
- ΔS o :
-
Standard entropy change, kJ mol−1
- ΔH o :
-
Standard enthalpy change, kJ mol−1 K−1
- K o :
-
Thermodynamic equilibrium constant, dimensionless
- R :
-
Universal constant, kJ mol−1 K−1
- T :
-
Temperature, K
- λ max :
-
Maximum wave number
- ρ p :
-
Particle density, kg m−3
- ρ S :
-
Solid density, kg m−3
- ε :
-
Void fraction
References
Al–Khateeb LA, Almotiry S, Salam MA (2014) Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem Eng J 248:191–199
Ali I, Al –Othman ZA, Alwarthan A, Assim M, Khan TA (2014) Removal of arsenic species from water by batch and column operations on bagasse fly ash. Env Sci Pollut Res 21:3218–3229
Ali I, Al –Othman ZA, Alwarthan A (2016) Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J Mol Liq 219:858–864
Anastopoulos I, Kyzas GZ (2016) Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? J Mol Liq 218:174–185
Baccar R, Sarrà M, Bouzid J, Feki M, Blánquez P (2012) Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J 211–212:310–317
Basheer AA (2018) New generation nano-adsorbents for the removal of emerging contaminants in water. J Mol Liq 261:583–593
Beltrame KK, Cazetta AL, de Souza PSC, Spessato L, Silva TL, Almeida VC (2018) Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol Environ Saf 147:64–71
Bergmann CP (2015) Carbon nanomaterials as adsorbents for environmental and biological applications
Bonilla-Petriciolet A, Mendoza-Castillo DI, Reynel-Avila HE (2017) Adsorption processes for water treatment and purification
Breitbach M, Bathen D (2001) Influence of ultrasound on adsorption processes. Ultrason Sonochem 8:277–283
Chen F, Ying GG, Kong LX, Wang L, Zhao JL, Zhou LJ, Zhang LJ (2011) Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ Pollut 159:1490–1498
Comber S, Gardner M, Sörme P, Leverett D, Ellor B (2017) Active pharmaceutical ingredients entering the aquatic environment from wastewater treatment works: a cause for concern? Sci Total Environ 613–614:538–547
Do DD (1998) Adsorption analysis: equilibria and kinetics
Dotto GL, Santos JMN, Rodrigues IL, Rosa R, Pavan FA, Lima EC (2015) Adsorption of methylene blue by ultrasonic surface modified chitin. J Colloid Interface Sci 446:133–140
Fabbri E (2015) Pharmaceuticals in the environment: expected and unexpected effects on aquatic fauna. Ann N Y Acad Sci 1340:20–28
Ferreira LS, Rodrigues MS, de Carvalho JCM, Lodi A, Finocchio E, Perego P, Converti A (2011) Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem Eng J 173:326–333
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem A 57:385–470
Franco DSP, Tanabe EH, Dotto GL (2017) Continuous adsorption of a cationic dye on surface modified rice husk: statistical optimization and dynamic models. Chem Eng Commun 204:625–634
Fröhlich AC, Ocampo-Pérez R, Diaz-Blancas V, Salau NPG, Dotto GL (2018) Three-dimensional mass transfer modeling of ibuprofen adsorption on activated carbon prepared by sonication. Chem Eng J 341:65–74
Gibs J, Heckathorn HA, Meyer MT, Klapinski FR, Alebus M, Lippincott RL (2013) Occurrence and partitioning of antibiotic compounds found in the water column and bottom sediments from a stream receiving two wastewater treatment plant effluents in Northern New Jersey, 2008. Sci Total Environ 458–460:107–116
Goldstein J, Newburry D, Joy D, Lyman C, Echlin P, Lifshin E, Sawyer L, Michael J (2003) Scanning electron microscopy and X-ray microanalysis, Third. edn. Kluwer Academic/Plenum Publishers, New York
González–Alonso S, Merino LM, Esteban S, López de Alda M, Barceló D, Durán JJ, López–Martínez J, Aceña J, Pérez S, Mastroianni N, Silva A, Catalá M, Valcárcel Y (2017) Occurrence of pharmaceutical, recreational and psychotropic drug residues in surface water on the northern Antarctic Peninsula region. Environ Pollut 229:241–254
Gupta VK, Ali I (2012) Environmental water: advances in treatment, remediation and recycling. Elsevier, Amsterdam
Hidayu AR, Muda N (2016) Preparation and characterization of impregnated activated carbon from palm kernel shell and coconut shell for CO2 capture. Procedia Eng 148:106–113
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Prot 76:183–191
Inglezakis VJ, Poulopoulos SG (2006) Adsorption, ion exchange and catalysis: design of operations and environmental applications Elsevier Sci B V 602
Iovino P, Canzano S, Capasso S, Erto A, Musmarra D (2015) A modeling analysis for the assessment of ibuprofen adsorption mechanism onto activated carbons. Chem Eng J 277:360–367
Katsigiannis A, Noutsopoulos C, Mantziaras J, Gioldasi M (2015) Removal of emerging pollutants through granular activated carbon. Chem Eng J 280:49–57
Kleywegt S, Pileggi V, Yang P, Hao C, Zhao X, Rocks C, Thach S, Cheung P, Whitehead B (2011) Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—occurrence and treatment efficiency. Sci Total Environ 409:1481–1488
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K Sven Vetensk 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Leyva-Ramos R, Ocampo-Perez R, Mendoza-Barron J (2012) External mass transfer and hindered diffusion of organic compounds in the adsorption on activated carbon cloth. Chem Eng J 183:141–151
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut 187:193–201
Montes-Grajales D, Fennix-Agudelo M, Miranda-Castro W (2017) Occurrence of personal care products as emerging chemicals of concern in water resources: a review. Sci Total Environ 595:601–614
Ocampo-Perez R, Aguilar-Madera CG, Díaz-Blancas V (2017) 3D modeling of overall adsorption rate of acetaminophen on activated carbon pellets. Chem Eng J 321:510–520
Sagheer FAA, Al– Sughayer MA, Muslim S, Elsabee MZ (2009) Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr Polym 77:410–419
Sellaoui L, Dotto GL, Gonçalves JO, Pinto LAA, Knani S, Lamine AB (2016) Equilibrium modeling of single and binary adsorption of Food Yellow 4 and Food Blue 2 on modified chitosan using a statistical physics theory: new microscopic interpretations. J Mol Liq 222:151–158
Sharma S, Bhattacharya A (2017) Drinking water contamination and treatment techniques. Appl Water Sci 7:1043–1067
Silverstein R, Webster X, Kiemle D (2005) Spectrometric identification of organic compounds
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Souza PR, Dotto GL, Salau NPG (2017) Detailed numerical solution of pore volume and surface diffusion model in adsorption systems. Chem Eng Res Des 122:298–307
Vannini C, Domingo G, Marsoni M, De Mattia F, Labra M, Castiglioni S, Bracale M (2011) Effects of a complex mixture of therapeutic drugs on unicellular algae Pseudokirchneriella subcapitata. Aquat Toxicol 101:459–465
von Oepen B, Kördel W, Klein W (1991) Sorption of nonpolar and polar compounds to soils: processes, measurements and experience with the applicability of the modified OECD-Guideline 106. Chemosphere 22:285–304
Yang K, Peng J, Srinivasakannan C, Zhang L, Xia H, Duan X (2010) Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour Technol 101:6163–6169
Acknowledgements
The authors would like to thank Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Electronic supplementary material
ESM 1
(DOCX 4269 kb)
Rights and permissions
About this article
Cite this article
Fröhlich, A.C., dos Reis, G.S., Pavan, F.A. et al. Improvement of activated carbon characteristics by sonication and its application for pharmaceutical contaminant adsorption. Environ Sci Pollut Res 25, 24713–24725 (2018). https://doi.org/10.1007/s11356-018-2525-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2525-x