Abstract
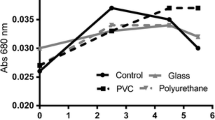
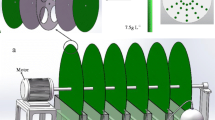
This article investigates the innovative attached cultivation of Chlorella vulgaris (C. vulgaris) using different materials as an alternative to high capital techniques of harvesting such as centrifugation, flocculation, and filtration. A simple attached algal cultivation system was proposed that was equipped by 10 submerged supporting materials which can harvest algal cells, efficiently. The effect of operational parameters such as light intensity, the rate of aeration, and auto-harvesting time was investigated. A chip, durable, and abundant cellulosic material (Kaldnes carriers covered by kenafs, KCCKs) was proposed for auto-harvesting C. vulgaris cells. The results revealed that optimum aeration rate, light intensity, and auto-harvesting of microalgal cells were 3.6 vvm, 10,548 W/m2, and 12 days, respectively. Six of these KCCKs had the highest biofilm formation percent up to 33%. In this condition, the rate of cell growth increased to 0.6 mg/cm2. Therefore, this system can be used for appropriate auto-harvesting of microalgae in the attached growth systems. C. vulgaris biomass composition is valuable for biodiesel, bioethanol, and animal protein production.








Similar content being viewed by others
References
Al-Qasmi M, Raut N, Talebi S, Al-Rajhi S, Al-Barwani T (2012) A review of effect of light on microalgae growth, Proceedings of the world congress on Engineering, 4–6
Alavijeh RS, Tabandeh F, Tavakoli O, Karkhane A, Shariati P (2015) Enzymatic production of biodiesel from microalgal oil using ethyl acetate as an acyl acceptor. J Oleo Sci 64:69–74
Atiku H, Mohamed R, Al-Gheethi A, Wurochekke A, Kassim AHM (2016) Harvesting of microalgae biomass from the phycoremediation process of greywater. Environ Sci Pollut Res 23:24624–24641
Bertoldi FC, Sant'Anna E, Oliveira JLB (2008) Chlorophyll Content And Minerals Profile In The Microalgae Chlorella Vulgaris Cultivated In Hydroponic Wastewater. Ciência Rural 38: 54–58.
Biller P, Ross A (2011) Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour Technol 102:215–225
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Brummer Y, Cui SW (2005) Understanding carbohydrate analysis. Food carbohydrates: chemistry, physical properties and applications:1–38. https://doi.org/10.1201/9780203485286.ch2
Chacón-Lee T, González-Mariño G (2010) Microalgae for “healthy” foods possibilities and challenges. Compr Rev Food Sci Food Saf 9:655–675
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee D-J, Chang J-S (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62
Connon R (2007) Culturing of Chlorella vulgaris—standard operating procedure. Daphnia Research group (University of Reading). Cited in: 2018.02.28. http://www.biosci.rdg.ac.uk/Research/eb/daphnia.htm
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect Of Temperature And Nitrogen Concentration On The Growth And Lipid Content Of Nannochloropsis Oculata And Chlorella Vulgaris For Biodiesel Production. Chem Eng Process. Process Intensif 48: 1146–1151.
Daliry S, Hallajsani A, Mohammadi Roshandeh J, Nouri H, Golzary A (2017) Investigation of optimal condition for Chlorella vulgaris microalgae growth. Global J Environ Sci Manag 3:217–230
da Silva MF, Casazza AA, Ferrari PF, Aliakbarian B, Converti A, Bezerra RP, Porto ALF, Perego P (2017) Recovery of phenolic compounds of food concern from Arthrospira platensis by green extraction techniques. Algal Res 25:391–401. https://doi.org/10.1016/j.algal.2017.05.027
Derakhshan Z, Mahvi AH, Ehrampoush MH, Ghaneian MT, Yousefinejad S, Faramarzian M, Mazloomi SM, Dehghani M, Fallahzadeh H (2018) Evaluation of kenaf fibers as moving bed biofilm carriers in algal membrane photobioreactor. Ecotoxicol Environ Saf 152:1–7. https://doi.org/10.1016/j.ecoenv.2018.01.024
Gao F, Yang Z-H, Li C, Zeng G-M, Ma D-H, Zhou L (2015) A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour Technol 179:8–12
Genin SN, Aitchison JS, Allen DG (2014) Design of algal film photobioreactors: material surface energy effects on algal film productivity, colonization and lipid content. Bioresour Technol 155:136–143
Green B, Durnford D (1996) The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Biol 47:685–714
Gressler V, Yokoya NS, Fujii MT, Colepicolo P, Mancini Filho J, Torres RP, Pinto E (2010) Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem 120:585–590
Griffiths MJ, Harrison ST (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427
Hoffmann JP (1998) Wastewater treatment with suspended and nonsuspended algae. J Phycol 34:757–763
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang Y, Xiong W, Liao Q, Fu Q, Xia A, Zhu X, Sun Y (2016) Comparison of Chlorella vulgaris biomass productivity cultivated in biofilm and suspension from the aspect of light transmission and microalgae affinity to carbon dioxide. Bioresour Technol 222:367–373
Hultberg M, Jönsson HL, Bergstrand K-J, Carlsson AS (2014) Impact of light quality on biomass production and fatty acid content in the microalga Chlorella vulgaris. Bioresour Technol 159:465–467
Illman A, Scragg A, Shales S (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635
John RP, Anisha G, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Johnson MB, Wen Z (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85:525–534
Jones OG (2016) Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogs. Curr Opin Food Sci 7:7–13
Kay RA, Barton LL (1991) Microalgae as food and supplement. Crit Rev Food Sci Nutr 30:555–573
Khoeyi ZA, Seyfabadi J, Ramezanpour Z (2012) Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac Int 20:41–49
Khoo CG, Lam MK, Lee KT (2016) Pilot-scale semi-continuous cultivation of microalgae Chlorella vulgaris in bubble column photobioreactor (BC-PBR): hydrodynamics and gas–liquid mass transfer study. Algal Res 15:65–76
Lee SH, Oh HM, Jo BH, Lee SA, Shin SY, Kim HS, Lee SH, Ahn CY (2014) Higher biomass productivity of microalgae in an attached growth system, using wastewater. J Microbiol Biotechnol 24:1566–1573
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced Lipid Production Of Chlorella Vulgaris By Adjustment Of Cultivation Conditions. Bioresour Technol 101: 6797–6804.
Mahdavi M, Mahvi AH, Pourzamani H, Fatehizadeh A, Ebrahimi A (2017) High turbid water treatment by Kenaf fibers: a practical method for individual water supply and remote areas. Desalin Water Treat 76:225–231
Månsson S (2012) Cultivation of Chlorella vulgaris in nutrient solution from greenhouse tomato production: a possibility to reduce nutrient levels and produce commercially interesting metabolites, Second cycle, A2E. Alnarp: SLU, Plant Breeding and Biotechnology (until 121231), sweden
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Milano J, Ong HC, Masjuki H, Chong W, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197
Petkov G, Garcia G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35:281–285
Phukan MM, Chutia RS, Konwar B, Kataki R (2011) Microalgae Chlorella as a potential bio-energy feedstock. Appl Energy 88:3307–3312
Pignolet O, Jubeau S, Vaca-Garcia C, Michaud P (2013) Highly valuable microalgae: biochemical and topological aspects. J Ind Microbiol Biotechnol 40:781–796
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Qin L, Wang Z, Sun Y, Shu Q, Feng P, Zhu L, Xu J, Yuan Z (2016) Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ Sci Pollut Res Int 23:8379–8387
Richmond A, Hu Q (2013) Handbook of microalgal culture: applied phycology and biotechnology. Wiley https://doi.org/10.1002/9781118567166
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278
Schnurr PJ, Allen DG (2015) Factors affecting algae biofilm growth and lipid production: a review. Renew Sust Energ Rev 52:418–429
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16
Tabatabaei M, Tohidfar M, Jouzani GS, Safarnejad M, Pazouki M (2011) Biodiesel production from genetically engineered microalgae: future of bioenergy in Iran. Renew Sust Energ Rev 15:1918–1927
Thomas WH, Tornabene TG, Weissman J (1984) Screening for lipid yielding microalgae: activities for 1983. Final subcontract report, Solar Energy Research Inst., Golden, CO (USA)
Tokuşoglu Ö, Üunal M (2003) Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J Food Sci 68:1144–1148
Waghmare AG, Salve MK, LeBlanc JG, Arya SS (2016) Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour Bioprocess 3:16
Yusof YAM, Basari JMH, Mukti NA, Sabuddin R, Muda AR, Sulaiman S, Makpol S, Ngah WZW (2011) Fatty acids composition of microalgae Chlorella vulgaris can be modulated by varying carbon dioxide concentration in outdoor culture. Afr J Biotechnol 10:13536–13542
Zhuang L-L, Hu H-Y, Wu Y-H, Wang T, Zhang T-Y (2014) A novel suspended-solid phase photobioreactor to improve biomass production and separation of microalgae. Bioresour Technol 153:399–402
Funding
This study is a part of an approved research project (no. 194075) performed at Isfahan University of Medical Sciences, Iran, and is funded by the Vice research of Isfahan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Jafari, N., Shafiee Alavijeh, R., Abdolahnejad, A. et al. An innovative approach to attached cultivation of Chlorella vulgaris using different materials. Environ Sci Pollut Res 25, 20097–20105 (2018). https://doi.org/10.1007/s11356-018-2177-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2177-x