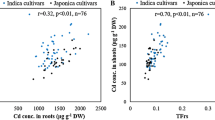
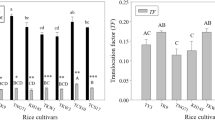
Abstract
Constant exposure of the living ecosystems to heavy metals, like cadmium (Cd), induces a detectable change at the biochemical and genetic level. Repeated application of phosphate fertilizers in paddy fields, leads to increase in Cd content of soil. Cd being highly mobile is transported to shoot and grain, thereby entering into the food chain of animal system. In the present study, treatment of 7-day old rice seedlings with 10 μM cadmium chloride resulted in Cd toxicity across the seven non-aromatic and six aromatic rice cultivars and landraces used for the study. Free proline and malondialdehyde content of treated samples were higher in comparison to the untreated samples, which indicated Cd induced tissue damage in plants. Photosynthetic pigment content of treated samples was also found to be much lower in comparison to the untreated samples, which is probably due to peroxidation of membrane, leading to compromised and lower photosynthetic efficiency of treated plants. At the genetic level, Randomly Amplified Polymorphic DNA assay was found to efficiently detect the genetic polymorphisms (caused by alterations in DNA bases) induced by Cd. Production of unique polymorphic bands in Cd-treated plants helps in assessment of the degree of damage Cd imparts on the plant system. Cluster analysis was performed and the rice genotypes were grouped into five distinct clusters, with IR64 and Tulsibhog in two distinct groups. Based on the variability in responses, the 13 rice genotypes were grouped into sensitive and tolerant ones.








Similar content being viewed by others
References
Aksoy KD, Aras S (2011) Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and population levels by use of various biomarkers. Mutat Res 719:29–34
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenol oxidases in Beta vulgaris. Plant Physiol 24:1–15
Aslam R, Bhat TM, Choudhury S, Ansari MYK (2017) An overview on genotoxicity of heavy metals in a spice crop (Capsicum annum L.) in respect to cyto-morphological behavior. Caryologia 70:42–47
Atienzar FA, Conradi M, Evenden AJ, Jha AN, Depledge MH (1999) Qualitative assessment of genotoxicity using random amplified polymorphic DNA: comparison of genomic template stability with key fitness parameters in Daphnia magna to benzo [a] pyrene. Environ Toxicol Chem 18:2275–2282
Atienzar FA, Cordi B, Donkin ME, Evenden AJ, Jha AN, Depledge MH (2000) Comparison of ultraviolet-induced genotoxicity detected by random amplified polymorphic DNA with chlorophyll fluorescence and growth in a marine macroalgae. Palmariapalmata. Aquat Toxicol 50:1–12
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Cao D, Zhang HZ, Wang YD, Zheng LN (2014) Accumulation and distribution characteristics of zinc and cd in the hyperaccumulator plant Sedum plumbizincicola. Bull Environ Contam Toxicol 93(2):171–176
Cenkçi S, Yıldız M, Cigerci İ H, Konuk M (2009) Toxic chemicals-induced genotoxicity detected by random amplified polymorphic DNA (RAPD) in bean (Phaseolus vulgaris L.) seedlings. Chemosphere 76:900–906
Cenkci S, Cigerci IH, Yildiz M, Ozay C, Bozdag A, Terzi H (2010) Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ Exp Bot 67:467–473
Ci D, Jiang D, Wollenweber B (2010) Genetic variance in Cd tolerance and accumulation in wheat materialsdiffering in ploidy and genome at seedling stage. J Agron Crop Sci 196:302–310
Daud MK, Sun Y, Dawood M (2009) Cd-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J Hazard Mater 161:463–473
Food and Agriculture Organization/World Health Organization (2010) Joint FAO/WHO Expert Committee on Food Additives, Seventy-Third Meeting, Geneva, 8–17June 2010. Summary and Conclusions. Available at: www.who.int/foodsafety/publications/chem/summary73.pdf
Gupta M, Sarin NB (2009) Heavy metal induced DNA changes in DNA analysis and identification of sequence characterized amplified region marker. J Environ Sci 21:686–690
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Snyder WC (1933) Nutrition of strawberry plants under controlled conditions: (a) effects of deficiencies of boron and certain other elements: (b) susceptibility to injury from sodium salts. Proceedings of the. Am Soc Horticult Sci 30:288–294
International Rice Research Institute (2011) World production and consumption of domestic milled rice. Available at: http://ricestat.irri.org/vis/wrs_quickCharts.php
Irfan M, Hayat S, Ahmad A, Alyemen MN (2013) Soil Cd enrichment: allocation and plant physiological manifestations. Saudi J Biol Sci 20:1–10
Ishikawa S, Ae N, Sugiyama M, Murakami M, Arao T (2005) Genotypic variation in shoot cadmium concentration in rice and soybean in soils with different levels of cadmium contamination. Soil Sci Plant Nutr 51:101–108
Koca H, Bor M, Ozdemir F, Turkan I (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Kosolsaksakul P, Farmer JG, Oliver IW, Graham MC (2014) Geochemical associations and availability of Cd (Cd) in a paddy field system, northwestern Thailand. Environ Pollut 187:153–161
Labra M, Di Fabio T, Grassi F, Regondi SM (2003) AFLP analysis as biomarker of exposure to organic and inorganic genotoxic substances in plants. Chemosphere 52:1183–1188
Liu W, Li P, Qi XM, Zhou Q (2005) DNA changes in bar-ley (Hordeum vulgare) seedlings induced by Cd pollution using RAPD analysis. Chemosphere 61:158–167
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Moradi A, Abbaspour KC, Afyuni M (2005) Modelling field-scale Cd transport below the root zone of a sewage sludgevamended soil in an arid region in Central Iran. J Contam Hydrol 79:187–206
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216
Roberts TL (2014) Cd and phosphorous fertilizers: the issues and the science. Procedia Eng 83:52–59
Rodriguez FM, Rodriguez CE (1982) Lead and Cd levels in soil and plants near highways and their correlation with traffic density. Environ Pollut Ser B 4:281–290
Saghai MA, Soliman KM, Jorgenson RA, Allard RW (1984) Ribosomal DNA spacer length polymorphism in barley, Mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci U S A 81:8014–8018
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Singh S, Eapen S, D’Souza SF (2006) Cd accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246
Sizova OI, Kochetkov VV, Validov SZ, Boronin AM, Kostcrin PV, Lyubun YV (2004) Arsenic contaminated soils: genetically modified Pseudomonas spp. and their arsenic phytoremediation potential. J. Soil Sediment 2:19–23
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 41:2930–2936
Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS (2014) Cd and lead interactive effects on oxidative stress andantioxidative responses in rice seedlings. Protoplasma 251:1047–1065
Suzuki N (2005) Alleviation by calcium of cd-induced root growth inhibition in Arabidopsis seedlings. Plant Biotechnol 22:19–25
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Yu Z, Zhou Q (2009) Growth responses and Cd accumulation of Mirabilis jalapa L. under interaction between Cd and phosphorus. J Hazard Mater 167:38–43
Zhang F, Zhang H, Wang G (2009) Cd-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J Hazard Mater 168:76–84
Acknowledgements
SM acknowledges Department of Biotechnology, Government of India, for the fellowship. The authors also hold deep sense of gratitude to Centre of Advanced Studies, Department of Botany, UGC-CAS Phase VII, University of Calcutta for the Research facilities. The authors are also thankful to Prof. S. Bhattacharya, Bidhan Chandra Krishi VishwaVidyalaya for the rice seed samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yi-ping Chen
Rights and permissions
About this article
Cite this article
Majumdar, S., Chakraborty, B. & Kundu, R. Comparative analysis of cadmium-induced stress responses by the aromatic and non-aromatic rice genotypes of West Bengal. Environ Sci Pollut Res 25, 18451–18461 (2018). https://doi.org/10.1007/s11356-018-1966-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1966-6