Abstract
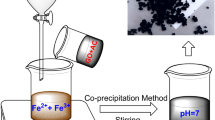
A superparamagnetic graphene oxide (GO)/Fe3O4 nanocomposite (MGO) was prepared by a facile in situ co-precipitation strategy, resulting in a prospective material for the application of graphene oxide in wastewater treatment. MGO was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), x-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR). The prepared adsorbent showed a high adsorption efficiency relevant to the purification of dye-contaminated wastewater and could be readily magnetically separated. The maximum adsorption capacity was ca. 546.45 mg g−1 for the common cationic dye methylene blue (MB) and ca. 628.93 mg g−1 for the anionic dye Congo red (CR). The adsorption processes fit the pseudo-second-order kinetic model well, which revealed that these processes may involve the chemical interaction between adsorbate and adsorbent. The thermodynamic parameters indicated that the adsorption reaction was an endothermic and spontaneous process. Furthermore, the prepared magnetic adsorbent had a wide effective pH range from 5 to 11 and showed good stability after five reuse cycles. The synthetic MGO showed great potential as a promising adsorbent for organic contaminant removal in wastewater treatment.






Similar content being viewed by others
References
Abidi N, Errais E, Duplay J, Berez A, Jrad A, Schaefer G, Ghazi M, Semhi K, Trabelsi-Ayadi M (2015) Treatment of dye-containing effluent by natural clay. J Clean Prod 86:432–440
Ahmad R, Kumar R (2010) Adsorptive removal of Congo red dye from aqueous solution using bael shell carbon. Appl Surf Sci 257:1628–1633
Akbari A, Remigy JC, Aptel P (2002) Treatment of textile dye effluent using a polyamide-based nanofiltration membrane. Chem Eng Process 41:601–609
Aliyari E, Alvand M, Shemirani F (2016) Modified surface-active ionic liquid-coated magnetic graphene oxide as a new magnetic solid phase extraction sorbent for preconcentration of trace nickel. RSC Adv 6:64193–64202
Barathi M, Kumar ASK, Kumar CU, Rajesh N (2014) Graphene oxide-aluminium oxyhydroxide interaction and its application for the effective adsorption of fluoride. RSC Adv 4:53711–53721
Belpaire C, Reyns T, Geeraerts C, Van Loco J (2015) Toxic textile dyes accumulate in wild European eel Anguilla anguilla. Chemosphere 138:784–791
Chang Y-H, Huang C-F, Hsu W-J, Chang F-C (2007) Removal of Hg2+ from aqueous solution using alginate gel containing chitosan. J Appl Polym Sci 104:2896–2905
Chen L, Li YH, Hu S, Sun JK, Du QJ, Yang XX, Ji Q, Wang ZH, Wang DC, Xia YZ (2016) Removal of methylene blue from water by cellulose/graphene oxide fibres. J Exp Nanosci 11:1156–1170
Cheng Z, Liao J, He B, Zhang F, Zhang F, Huang X, Zhou L (2015) One-step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. ACS Sustain Chem Eng 3:1677–1685
Cheng ZL, Li W, Wu PR, Liu Z (2017) A strategy for preparing modified graphene oxide with good dispersibility and transparency in oil. Ind Eng Chem Res 56:5527–5534
Dawood SS, Sen TK (2012) Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res 46:1933–1946
Demirbas E, Dizge N, Sulak MT, Kobya M (2009) Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem Eng J 148:480–487
Dizaji AK, Mortaheb HR, Mokhtarani B (2016) Noncovalently functionalized graphene oxide/graphene with imidazolium-based ionic liquids for adsorptive removal of dibenzothiophene from model fuel. J Mater Sci 51:10092–10103
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Du Q, Sun J, Li Y, Yang X, Wang X, Wang Z, Xia L (2014) Highly enhanced adsorption of Congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem Eng J 245:99–106
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Greenwald MJ, Redding AM, Cannon FS (2015) A rapid kinetic dye test to predict the adsorption of 2-methylisoborneol onto granular activated carbons and to identify the influence of pore volume distributions. Water Res 68:784–792
Guo YF, Deng J, Zhu JY, Zhou C, Zhou CY, Zhou XJ, Bai RB (2016) Removal of anionic azo dye from water with activated graphene oxide: kinetic, equilibrium and thermodynamic modeling. RSC Adv 6:39762–39773
Halouane F, Oz Y, Meziane D, Barras A, Juraszek J, Singh SK, Kurungot S, Shaw PK, Sanyal R, Boukherroub R, Sanyal A, Szunerits S (2017) Magnetic reduced graphene oxide loaded hydrogels: highly versatile and efficient adsorbents for dyes and selective Cr(VI) ions removal. J Colloid Interf Sci 507:360–369
Hanafiah M, Ngah WSW, Zolkafly SH, Teong LC, Majid ZAA (2012) Acid blue 25 adsorption on base treated Shorea dasyphylla sawdust: kinetic, isotherm, thermodynamic and spectroscopic analysis. J Environ Sci 24:261–268
Ho YS (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40:119–125
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ 76:183–191
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Huang GJ, Chen ZG, Li MD, Yang B, Xin ML, Li SP, Yin ZJ (2016) Surface functional modification of graphene and graphene oxide. Acta Chim Sin 74:789–799
Kyzas GZ, Fu J, Matis KA (2013) The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials 6:5131–5158
Liu T, Li Y, Du Q, Sun J, Jiao Y, Yang G, Wang Z, Xia Y, Zhang W, Wang K, Zhu H, Wu D (2012) Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf B Biointerfaces 90:197–203
Lucas MS, Peres JA (2006) Decolorization of the azo dye reactive black 5 by Fenton and photo-Fenton oxidation. Dyes Pigments 71:236–244
Ma X, Chen PL, Zhou M, Zhong ZX, Zhang F, Xing WH (2017) Tight ultrafiltration ceramic membrane for separation of dyes and mixed salts (both NaCl/Na2SO4) in textile wastewater treatment. Ind Eng Chem Res 56:7070–7079
Ma H, Pu S, Ma J, Yan C, Zinchenko A, Pei X, Chu W (2018) Formation of multi-layered chitosan honeycomb spheres via breath-figure-like approach in combination with co-precipitation processing. Mater Lett 211:91–95
Malachova K, Rybkova Z, Sezimova H, Cerven J, Novotny C (2013) Biodegradation and detoxification potential of rotating biological contactor (RBC) with Irpex lacteus for remediation of dye-containing wastewater. Water Res 47:7143–7148
Netpradit S, Thiravetyan P, Towprayoon S (2003) Application of ‘waste’ metal hydroxide sludge for adsorption of azo reactive dyes. Water Res 37:763–772
Peng R, Chen X, Ghosh R (2017a) Preparation of graphene oxide-cotton fiber composite adsorbent and its application for the purification of polyphenols from pomegranate peel extract. Sep Purif Technol 174:561–569
Peng W, Li H, Liu Y, Song S (2017b) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504
Pu S, Xiang C, Zhu R, Ma H, Zinchenko A, Chu W (2017a) An efficient heterogeneous Fenton catalyst based on modified diatomite for degradation of cationic dye simulated wastewater. Desalin Water Treat 79:378–385
Pu S, Zhu R, Ma H, Deng D, Pei X, Qi F, Chu W (2017b) Facile in-situ design strategy to disperse TiO2 nanoparticles on graphene for the enhanced photocatalytic degradation of rhodamine 6G. Applied Catalysis B-Environmental 218:208–219
Pu SY, Ma H, Zinchenko A, Chu W (2017d) Novel highly porous magnetic hydrogel beads composed of chitosan and sodium citrate: an effective adsorbent for the removal of heavy metals from aqueous solutions. Environ Sci Pollut R 24:16520–16530
Ren Y, Abbood HA, He F, Peng H, Huang K (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311
Riera-Torres M, Gutierrez-Bouzan MC, Morell JV, Lis MJ, Crespi M (2011) Influence of electrochemical pre-treatment in dyeing wastewater reuse for five reactive dyes. Text Res J 81:1926–1939
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286
Szpyrkowicz L, Juzzolino C, Kaul SN (2001) A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and Fenton reagent. Water Res 35:2129–2136
Tang ZH, Shen SL, Zhuang J, Wang X (2010) Noble-metal-promoted three-dimensional macroassembly of single-layered graphene oxide. Angew Chem Int Edit 49:4603–4607
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interf Sci 286:90–100
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo red adsorption by clay materials. Chem Eng J 148:354–364
Wan Z, Wang JL (2017) Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J Hazard Mater 324:653–664
Wang H, Yuan XZ, Wu Y, Chen XH, Leng LJ, Wang H, Li H, Zeng GM (2015) Facile synthesis of polypyrrole decorated reduced graphene oxide-Fe3O4 magnetic composites and its application for the Cr(VI) removal. Chem Eng J 262:597–606
Wang F, Zhang LJ, Wang YY, Liu XJ, Rohani S, Lu J (2017) Fe3O4@SiO2@CS-TETA functionalized graphene oxide for the adsorption of methylene blue (MB) and cu(II). Appl Surf Sci 420:970–981
Wu Y, Luo HJ, Wang H (2014) Efficient removal of Congo red from aqueous solutions by surfactant-modified Hydroxo aluminum/graphene composites. Sep Sci Technol 49:2700–2710
Wu YC, Qi HJ, Shi C, Ma RX, Liu SX, Huang ZH (2017) Preparation and adsorption behaviors of sodium alginate-based adsorbent-immobilized beta-cyclodextrin and graphene oxide. RSC Adv 7:31549–31557
Yang CH, Wang CY, Huang KS, Yeh CS, Wang AHJ, Wang WT, Lin MY (2012): Facile synthesis of radial-like macroporous superparamagnetic chitosan spheres with in-situ co-precipitation and gelation of Ferro-gels. PLoS One 7, 7
Yao YJ, Miao SD, Liu SZ, Ma LP, Sun HQ, Wang SB (2012) Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem Eng J 184:326–332
Zhang WJ, Zhou CJ, Zhou WC, Lei AH, Zhang QL, Wan Q, Zou BS (2011) Fast and considerable adsorption of methylene blue dye onto graphene oxide. Bull Environ Contam Toxicol 87:86–90
Zhang LY, Zhang W, Zhou Z, Li CM (2016) Gamma-Fe2O3 nanocrystals-anchored macro/meso-porous graphene as a highly efficient adsorbent toward removal of methylene blue. J Colloid Interface Sci 476:200–205
Acknowledgments
The authors thank Prof. Anatoly Zinchenko for a helpful discussion.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 41772264, 51408074) and the Research Fund of State Key Laboratory of Geohazard Prevention and Geoenvironment Protection (SKLGP2017Z009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Responsible editor: Guilherme L. Dotto
Electronic supplementary material
ESM 1
(DOCX 536 kb)
Rights and permissions
About this article
Cite this article
Pu, S., Xue, S., Yang, Z. et al. In situ co-precipitation preparation of a superparamagnetic graphene oxide/Fe3O4 nanocomposite as an adsorbent for wastewater purification: synthesis, characterization, kinetics, and isotherm studies. Environ Sci Pollut Res 25, 17310–17320 (2018). https://doi.org/10.1007/s11356-018-1872-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1872-y