Abstract
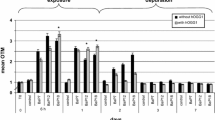
Diamondoids are polycyclic saturated hydrocarbons that possess a cage-like carbon skeleton approaching that of diamond. These ‘nano-diamonds’ are used in a range of industries including nanotechnologies and biomedicine. Diamondoids were thought to be highly resistant to degradation, but their presumed degradation acid products have now been found in oil sands process-affected waters (OSPW) and numerous crude oils. Recently, a diamondoid-related structure, 3-noradamantane carboxylic acid, was reported to cause genetic damage in trout hepatocytes under in vitro conditions. This particular compound has never been reported in the environment but led us to hypothesise that other more environmentally relevant diamondoid acids could also be genotoxic. We carried out in vivo exposures (3 days, semi-static) of marine mussels to two environmentally relevant diamondoid acids, 1-adamantane carboxylic acid and 3,5-dimethyladamantane carboxylic acid plus 3-noradamantane carboxylic acid with genotoxic damage assessed using the Comet assay. An initial screening test confirmed that these acids displayed varying degrees of genotoxicity to haemocytes (increased DNA damage above that of controls) when exposed in vivo to a concentration of 30 μmol L−1. In a further test focused on 1-adamantane carboxylic acid with varying concentrations (0.6, 6 and 30 μmol L−1), significant (P < 0.05 %) DNA damage was observed in different target cells (viz. gills and haemocytes) at 0.6 μmol L−1. Such a level of induced genetic damage was similar to that observed following exposure to a known genotoxin, benzo(a)pyrene (exposure concentration, 0.8 μmol L−1). These findings may have implications for a range of worldwide industries including oil extraction, nanotechnology and biomedicine.



Similar content being viewed by others
References
Banni M, Negri A, Dagnino A, Jebali J, Ameur S, Boussetta H (2010) Acute effects of benzo a pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicol Environ Saf 73:842–848. doi:10.1016/j.ecoenv.2009.12.032
Bayne BL, Widdows J, Moore MN, Salkeld P, Worrall CM, Donkin P (1982) Some ecological consequences of the physiological and biochemical effects of petroleum compounds on marine mollusks. Philos Trans R Soc Lond B Biol Sci 297:219–239
Booth AM et al (2007) Unresolved complex mixtures of aromatic hydrocarbons: thousands of overlooked persistent, bioaccumulative, and toxic contaminants in mussels. Environ Sci Technol 41:457–464
Canty MN, Hutchinson TH, Brown RJ, Jones MB, Jha AN (2009) Linking genotoxic responses with cytotoxic and behavioural or physiological consequences: differential sensitivity of echinoderms (Asterias rubens) and marine molluscs (Mytilus edulis). Aquat Toxicol 94:68–76. doi:10.1016/j.aquatox.2009.06.001
Collins AR (2014) Measuring oxidative damage to DNA and its repair with the comet assay. Biochim Biophys Acta 1840:794–800. doi:10.1016/j.bbagen.2013.04.022
Dallas LJ, Bean TP, Turner A, Lyons BP, Jha AN (2013) Oxidative DNA damage may not mediate Ni-induced genotoxicity in marine mussels: assessment of genotoxic biomarkers and transcriptional responses of key stress genes. Mutat Res Genet Toxicol Environ Mutagen 754:22–31. doi:10.1016/j.mrgentox.2013.03.009
Di Y, Schroeder DC, Highfield A, Readman JW, Jha AN (2011) Tissue-specific expression of p53 and ras genes in response to the environmental genotoxicant benzo(alpha)pyrene in marine mussels. Environ Sci Technol 45:8974–8981. doi:10.1021/es201547x
Dixon DR, Pruski AM, Dixon LRJ, Jha AN (2002) Marine invertebrate eco-genotoxicology: a methodological overview. Mutagenesis 17:495–507
Donkin P, Smith EL, Rowland SJ (2003) Toxic effects of unresolved complex mixtures of aromatic hydrocarbons accumulated by mussels, Mytilus edulis, from contaminated field sites. Environ Sci Technol 37:4825–4830
Frank RA et al. (2014) Profiling oil sands mixtures from industrial developments and natural groundwaters for source identification. Environ Sci Technol. doi:10.1021/es500131k
Gagné F et al (2012) Differential changes in gene expression in rainbow trout hepatocytes exposed to extracts of oil sands process-affected water and the Athabasca River. Comp Biochem Physiol C Toxicol Pharmacol 155:551–559. doi:10.1016/j.cbpc.2012.01.004
Gagné F, André C, Turcotte P, Gagnon C, Sherry J, Talbot A (2013) A comparative toxicogenomic investigation of oil sand water and processed water in rainbow trout hepatocytes. Arch Environ Contam Toxicol 65:309–323. doi:10.1007/s00244-013-9888-2
He YH et al (2011) Effect of ozonation on the estrogenicity and androgenicity of oil sands process-affected water. Environ Sci Technol 45:6268–6274. doi:10.1021/es2008215
He Y, Wiseman SB, Wang N, Perez-Estrada LA, El-Din MG, Martin JW, Giesy JP (2012) Transcriptional responses of the brain–gonad–liver axis of fathead minnows exposed to untreated and ozone-treated oil sands process-affected water. Environ Sci Technol 46:9701–9708. doi:10.1021/es3019258
Hook SE, Lee RF (2004) Genotoxicant induced DNA damage and repair in early and late developmental stages of the grass shrimp Paleomonetes pugio embryo as measured by the comet assay. Aquat Toxicol 66:1–14. doi:10.1016/j.aquatox.2003.06.002
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221. doi:10.1093/mutage/gen014
Jones D, Scarlett AG, West CE, Rowland SJ (2011) The toxicity of individual naphthenic acids to Vibrio fischeri. Environ Sci Technol 45:9776–9782. doi:10.1021/es201948j
Kavanagh RJ et al (2011) Fathead minnow (Pimephales promelas) reproduction is impaired in aged oil sands process-affected waters. Aquat Toxicol 101:214–220. doi:10.1016/j.aquatox.2010.09.021
Knag AC, Verhaegen S, Ropstad E, Mayer I, Meier S (2013) Effects of polar oil related hydrocarbons on steroidogenesis in vitro in H295R cells. Chemosphere 92:106–115. doi:10.1016/j.chemosphere.2013.02.046
Kumaravel TS, Jha AN (2006) Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res Genet Toxicol Environ Mutagen 605:7–16. doi:10.1016/j.mrgentox.2006.03.002
Kwok A, Lyons BP, Hodges NJ, Bean TP (2013) Cryopreservation and storage of mussel (Mytilus spp.) haemocytes for latent analysis by the Comet assay. Mutat Res Genet Toxicol Environ Mutagen 750:86–91. doi:10.1016/j.mrgentox.2012.09.010
Lacaze E, Devaux A, Bruneau A, Bony S, Sherry J, Gagné F (2014) Genotoxic potential of several naphthenic acids and a synthetic oil sands process-affected water in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 152:291–299. doi:10.1016/j.aquatox.2014.04.019
Landis WG, Chapman PM (2011) Well past time to stop using NOELs and LOELs Integrated Environ Assess Manag 7:vi-viii doi:10.1002/ieam.249
Lengger SK, Scarlett AG, West CE, Rowland SJ (2013) Diamondoid diacids (‘O4’ species) in oil sands process-affected water. Rapid Commun Mass Spectrom 27:2648–2654. doi:10.1002/rcm.6729
Lengger SK, Scarlett AG, West CE, Frank RA, Hewitt LM, Milestone CB, Rowland SJ (2015) Use of the distributions of adamantane acids to profile short-term temporal and pond-scale spatial variations in the composition of oil sands process-affected waters. Environmental Science: Processes & Impacts doi:10.1039/C5EM00287G
Mansoori GA, de Araujo PLB, de Araujo ES (2012) Diamondoid molecules: with applications in biomedicine, materials science, nanotechnology & petroleum science. World Scientific Publishing Co. Pte. Ltd, Singapore
Mitchelmore CL, Birmelin C, Chipman JK, Livingstone DR (1998) Evidence for cytochrome P-450 catalysis and free radical involvement in the production of DNA strand breaks by benzo a pyrene and nitroaromatics in mussel (Mytilus edulis L.) digestive gland cells. Aquat Toxicol 41:193–212. doi:10.1016/s0166-445x(97)00083-0
Peters LE, MacKinnon M, Van Meer T, van den Heuvel MR, Dixon DG (2007) Effects of oil sands process-affected waters and naphthenic acids on yellow perch (Perca flavescens) and Japanese medaka (Orizias latipes) embryonic development. Chemosphere 67:2177–2183. doi:10.1016/j.chemosphere.2006.12.034
Reinardy HC, Scarlett AG, Henry TB, West CE, Hewitt LM, Frank RA, Rowland SJ (2013) Aromatic naphthenic acids in oil sands process-affected water, resolved by GCxGC-MS, only weakly induce the gene for vitellogenin production in zebrafish (Danio rerio) larvae. Environ Sci Technol 47:6614–6620. doi:10.1021/es304799m
Rowland S, Donkin P, Smith E, Wraige E (2001) Aromatic hydrocarbon “humps” in the marine environment: unrecognized toxins? Environ Sci Technol 35:2640–2644
Rowland SJ, Scarlett A, West C, Jones D, Frank R (2011a) Diamonds in the rough: identification of individual naphthenic acids in oil sands process water. Environ Sci Technol 45:3154–3159. doi:10.1021/es103721b
Rowland SJ, West CE, Scarlett AG, Jones D (2011b) Identification of individual acids in a commercial sample of naphthenic acids from petroleum by two-dimensional comprehensive gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 25:1741–1751. doi:10.1002/rcm.5040
Rowland SJ, West CE, Scarlett AG, Jones D, Frank RA (2011c) Identification of individual tetra- and pentacyclic naphthenic acids in oil sands process water by comprehensive two-dimensional gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 25:1198–1204. doi:10.1002/rcm.4977
Rowland SJ, West CE, Scarlett AG, Ho C, Jones D (2012) Differentiation of two industrial oil sands process-affected waters by two-dimensional gas chromatography/mass spectrometry of diamondoid acid profiles. Rapid Commun Mass Spectrom 26:572–576. doi:10.1002/rcm.6138
Sansom B, Vo NTK, Kavanagh R, Hanner R, MacKinnon M, Dixon DG, Lee LEJ (2013) Rapid assessment of the toxicity of oil sands process-affected waters using fish cell lines. In Vitro Cell Dev Biol-Anim 49:52–65. doi:10.1007/s11626-012-9570-4
Scarlett AG, Clough R, West C, Lewis CA, Booth AM, Rowland SJ (2011) Alkylnaphthalenes: priority pollutants or minor contributors to the poor health of marine mussels? Environ Sci Technol 45:6160–6166. doi:10.1021/es201234a
Scarlett AG, West CE, Jones D, Galloway TS, Rowland SJ (2012) Predicted toxicity of naphthenic acids present in oil sands process-affected waters to a range of environmental and human endpoints. Sci Total Environ 425:119–127. doi:10.1016/j.scitotenv.2012.02.064
Scarlett AG, Reinardy HC, Henry TB, West CE, Frank RA, Hewitt LM, Rowland SJ (2013) Acute toxicity of aromatic and non-aromatic fractions of naphthenic acids extracted from oil sands process-affected water to larval zebrafish. Chemosphere 93:415–420. doi:10.1016/j.chemosphere.2013.05.020
Thomas KV, Langford K, Petersen K, Smith AJ, Tollefsen KE (2009) Effect-directed identification of naphthenic acids as important in vitro xeno-estrogens and anti-androgens in North Sea offshore produced water discharges. Environ Sci Technol 43:8066–8071. doi:10.1021/es9014212
Tung EWY, Philbrook NA, Belanger CL, Ansari S, Winn LM (2014) Benzo a pyrene increases DNA double strand break repair in vitro and in vivo: A possible mechanism for benzo a pyrene-induced toxicity. Mutat Res Genet Toxicol Environ Mutagen 760:64–69. doi:10.1016/j.mrgentox.2013.12.003
Vandenberg LN et al (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33:378–455. doi:10.1210/er.2011-1050
Villela IV, de Oliveira IM, da Silva J, Henriques JAP (2006) DNA damage and repair in haemolymph cells of golden mussel (Limnoperna fortunei) exposed to environmental contaminants. Mutat Res Genet Toxicol Environ Mutagen 605:78–86. doi:10.1016/j.mrgentox.2006.02.006
West CE, Scarlett AG, Pureveen J, Tegelaar EW, Rowland SJ (2013) Abundant naphthenic acids in oil sands process-affected water: studies by synthesis, derivatisation and two-dimensional gas chromatography/high-resolution mass spectrometry. Rapid Commun Mass Spectrom 27:357–365. doi:10.1002/rcm.6452
Acknowledgments
Funding for this study was provided by the European Research Council via an Advanced Investigators Award to Professor S. Rowland for project ‘OUTREACH’ (agreement no. 228149).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Dissanayake, A., Scarlett, A.G. & Jha, A.N. Diamondoid naphthenic acids cause in vivo genetic damage in gills and haemocytes of marine mussels. Environ Sci Pollut Res 23, 7060–7066 (2016). https://doi.org/10.1007/s11356-016-6268-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6268-2