Abstract
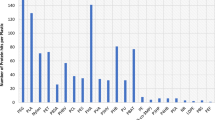
Hydrocarbon catabolic genes were investigated in soils and sediments in nine different locations around Syowa Station, Antarctica, using conventional PCR, real-time PCR, cloning, and sequencing analysis. Polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenase (PAH-RHD)-coding genes from both Gram-positive and Gram-negative bacteria were observed. Clone libraries of Gram-positive RHD genes were related to (i) nidA3 of Mycobacterium sp. py146, (ii) pdoA of Terrabacter sp. HH4, (iii) nidA of Diaphorobacter sp. KOTLB, and (iv) pdoA2 of Mycobacterium sp. CH-2, with 95–99 % similarity. Clone libraries of Gram-negative RHD genes were related to the following: (i) naphthalene dioxygenase of Burkholderia glathei, (ii) phnAc of Burkholderia sartisoli, and (iii) RHD alpha subunit of uncultured bacterium, with 41–46 % similarity. Interestingly, the diversity of the Gram-positive RHD genes found around this area was higher than those of the Gram-negative RHD genes. Real-time PCR showed different abundance of dioxygenase genes between locations. Moreover, the PCR-denaturing gradient gel electrophoresis (DGGE) profile demonstrated diverse bacterial populations, according to their location. Forty dominant fragments in the DGGE profiles were excised and sequenced. All of the sequences belonged to ten bacterial phyla: Proteobacteria, Actinobacteria, Verrucomicrobia, Bacteroidetes, Firmicutes, Chloroflexi, Gemmatimonadetes, Cyanobacteria, Chlorobium, and Acidobacteria. In addition, the bacterial genus Sphingomonas, which has been suggested to be one of the major PAH degraders in the environment, was observed in some locations. The results demonstrated that indigenous bacteria have the potential ability to degrade PAHs and provided information to support the conclusion that bioremediation processes can occur in the Antarctic soils and sediments studied here.




Similar content being viewed by others
References
Abell GCJ, Bowman JP (2005) Ecological and biogeographic relationships of class Flavobacteria in the Southern Ocean. FEMS Microbiol Ecol 51:265–277
Aislabie J, Foght JM (2010) Response of polar soil bacterial communities to fuel spills. In: Bej AK, Aislabie J, Atlas RM (eds) The ecology, biodiversity and bioremediation potential of microorganisms in extremely cold environments, Taylor & Francis, Florida, pp 215–230
Aislabie J, Foght J, Saul D (2000) Aromatic hydrocarbon-degrading bacteria from soil near Scott Base, Antarctica. Polar Biol 23:183–188
Aislabie JM, Balks MR, Foght JM, Waterhouse EJ (2004) Hydrocarbon spills on Antarctic soils: effects and management. Environ Sci Technol 38:1265–1274
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Mol Biol Rev 59:143–149
Arensköter M, Broker D, Steinbüchel A (2004) Biology of metabolically diverse genus Gordonia. Appl Environ Microbiol 70:3195–3204
Cébron A, Norini MP, Beguiristain T, Leyval C (2008) Real-time PCR quantification of PAH-ring hydroxylating dioxygenase (PAH-RHDalpha) genes from Gram positive and Gram negative bacteria in soil and sediment samples. J Microbiol Meth 73:148–159
Churchill PF, Morgan AC, Kitchens E (2008) Characterization of a pyrene-degrading Mycobacterium sp. strain CH-2. J Environ Sci Health B 43:698–706
Cunliffe M, Kawasaki A, Fellows E, Kertesz MA (2006) Effect of inoculum pretreatment on survival, activity and catabolic gene expression of Sphingobium yanoikuyae B1 in aged polycyclic aromatic hydrocarbon-contaminated soil. FEMS Microbiol Ecol 58:364–372
Das R, Kazy SK (2014) Microbial diversity, community composition and metabolic potential in hydrocarbon contaminated oily sludge: prospects for in situ bioremediation. Environ Sci Pollut Res. doi:10.1007/s1135601426402
DeBruyn JM, Chewning CS, Sayler GS (2007) Comparative quantitative prevalence of Mycobacteria and functionally abundant nidA, nahAc and nagAc dioxygenase genes in coal tar contaminated sediments. Environ Sci Technol 41:5426–5432
Delille D, Coulon F (2008) Comparative mesocosm study of biostimulation efficiency in two different oil-amended sub-Antarctic soils. Microb Ecol 56:243–252
Fernández-Luqueño F, Valenzuela-Encinas C, Marsch R, Martínez-Suárez C, Vázquez-Núñez E, Dendooven L (2011) Microbial communities to mitigate contamination of PAHs in soil—possibilities and challenges: a review. Environ Sci Pollut Res 18:12–30
Flocco CG, Gomes NCM, Cormack WM, Smalla K (2009) Occurrence and diversity of naphthalene dioxygenase genes in soil microbial communities from the Maritime Antarctic. Environ Microbiol 11:700–714
Gauthier MJ, Lafay B, Christen T, Fernandez L, Acquaviva M, Bonin PC, Betrand JC (1992) Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int J Syst Bacteriol 43:568–576
Hiraishi A, Yonemitsu Y, Matsushita M, Shin YK, Kuraishi H, Kawahara K (2002) Characterization of Porphyrobacter sanguineus sp. nov., an aerobic bacteriochlorophyll-containing bacterium capable of degrading biphenyl and dibenzofuran. Arch Microbiol 178:45–52
Junca H, Pieper DH (2004) Functional gene diversity analysis in BTEX contaminated soils by means of PCR-SSCP DNA fingerprinting: comparative diversity assessment against bacterial isolates and PCR-DNA clone libraries. Environ Microbiol 6:95–110
Jurelevicius D, Alvarez VM, Peixoto R, Rosado AS, Seldin L (2012a) Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases (PAH-RHD) encoding genes in different soils from King George Bay, Antarctic Peninsula. Appl Soil Ecol 55:1–9
Jurelevicius D, Cotta SR, Peixoto R, Rosado AS, Seldin L (2012b) Distribution of alkane-degrading bacterial communities in soils from King George Island, Maritime Antarctic. Eur J Soil Biol 51:37–44
Klankeo P, Nopcharoenkul W, Pinyakong O (2009) Two novel pyrene-degrading Diaphorobacter sp. and Pseudomonas sp. isolated from soil. J Biosci Bioeng 108:488–495
Kweon O, Kim SJ, Freeman JP, Song J, Baek S, Cerniglia CE (2010) Substrate specificity and structural characteristics of the novel Rieske non heme iron aromatic ring-hydroxylating oxygenases NidAB and NidA3B3 from Mycobacterium vanbaalenii PYR-1. mBio 1:1–11
Lau EV, Gan S, Ng HK (2010) Extraction techniques for polyaromatic hydrocarbons in soils. Int J Anal Chem. doi:10.1155/2010/398381
Laurie AD, Lloyd-Jones G (1999) The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism. J Bacteriol 181:531–540
Leys NM, Ryngaert A, Bastiaens L, Verstraete W, Springael D (2004) Occurrence and phylogenetic diversity of Sphingomonas strain in soils contaminated with polycyclic aromatic hydrocarbons. Appl Environ Microbiol 70:1944–1955
Liu C, Chen CX, Zhang XY, Yu Y, Liu A, Li GW, Chen XL, Chen B, Zhou BC, Zhang YZ (2012) Marinobacter antarcticus sp. nov., a halotolerant bacterium isolated from Antarctic intertidal sandy sediment. Int J Syst Evol Microbiol 62:1838–1844
Ma Y, Wang L, Shao Z (2006) Pseudomonas, the dominant polycyclic aromatic hydrocarbon-degrading bacteria isolated from Antarctic soils and the role of large plasmids in horizontal gene transfer. Environ Microbiol 8:455–465
Marcos MS, Lozada M, Dionisi HM (2009) Aromatic hydrocarbon degradation genes from chronically polluted Subantarctic marine sediments. Lett Appl Microbiol 49:602–608
Margesin R, Labbé D, Schinner F, Greer CW, Whyte LG (2003) Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl Environ Microbiol 69:3085–3092
Muangchinda C, Pansri R, Wongwongsee W, Pinyakong O (2013) Assessment of polycyclic aromatic hydrocarbon biodegradation potential in mangrove sediment from Don Hoi Lot, Samut Songkram Province, Thailand. J Appl Microbiol 114:1311–1324
Panicker G, Mojib N, Aislabie J, Bej AK (2010) Detection, expression and quantitation of the biodegradative genes in Antarctic microorganisms using PCR. Antonie Van Leeuwenhoek 97:275–287
Peng RH, Xiong AS, Xue Y, Fu XY, Gao F, Zhao W, Tian YS, Yao QH (2008) Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev 32:927–955
Powell SM, Bowman JP, Snape I, Stark JS (2003) Microbial community variation in pristine and polluted nearshore Antarctic sediments. FEMS Microbiol Ecol 45:135–145
Ruberto L, Vazquez SC, Mac Cormack WP (2003) Effectiveness of the natural bacterial flora, biostimulation and bioaugmentation on the bioremediation of a hydrocarbon contaminated Antarctic soil. Int Biodeterior Biodegrad 52:115–125
Ruberto LAM, Vazquez SC, Curtosi A, Mestre MC, Pelletier E, Mac Cormack WP (2006) Phenanthrene biodegradation in soils using an Antarctic bacterial consortium. Biorem J 10:191–201
Sipila TP, Riisio H, Yrjala K (2006) Novel upper meta-pathway extradiol dioxygenase gene diversity in polluted soil. FEMS Microbiol Ecol 58:134–144
Suenaga H, Mizuta S, Miyazaki K (2009) The molecular basis for adaptive evolution in novel extradiol dioxygenases retrieved from the metagenome. FEMS Microbiol Ecol 69:472–480
US EPA Method 3500C (2007) Organic extraction and sample preparation. Revision 3. February, 2007
US EPA Method 3540C (1996) Soxhlet extraction. Revision 3. December, 1996
US EPA Method 8310 (1986) Polyaromatic hydrocarbons. September, 1986
Yergeau E, Arbour M, Brousseau R, Juck D, Lawrence JR, Masson L, Whyte LG, Greer CW (2009) Microarray and real-time PCR analyses of the responses of high-arctic soil bacteria to hydrocarbon pollution and bioremediation treatments. Appl Environ Microbiol 75:6258–6267
Yergeau E, Bokhorst S, Huiskes AHL, Boschker HTS, Aerts R, Kowalchuk GA (2007) Size and structure of bacterial, fungal and nematode communities along an Antarctic environmental gradient. FEMS Microbiol Ecol 59:436–451
Zhang S, Wan R, Wang Q, Xie S (2011) Identification of anthracene degraders in leachate-contaminated aquifer using stable isotope probing. Int Biodeterior Biodegrad 65:1224–1228
Zhou HW, Guo CL, Wong YS, Tam NFY (2006) Genetic diversity of dioxygenase genes in PAH-degrading bacteria isolated from mangrove sediments. FEMS Microbiol Lett 262:148–157
Acknowledgments
This work was supported by the National Institute of Polar Research (Japan); L’Oreal (Thailand) Ltd.; Faculty of Science, Chulalongkorn University; and National Research University Project of the Office of Commission for Higher Education and Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University—Climate Change Cluster (CC1043A). We also would like to thank all the members of the 51st Japanese Antarctic Research Expedition (JARE-51) for their field support and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Muangchinda, C., Chavanich, S., Viyakarn, V. et al. Abundance and diversity of functional genes involved in the degradation of aromatic hydrocarbons in Antarctic soils and sediments around Syowa Station. Environ Sci Pollut Res 22, 4725–4735 (2015). https://doi.org/10.1007/s11356-014-3721-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3721-y