Abstract
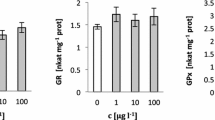
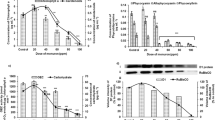
Cyanobacterial biofertilizers are affected by paddy field pesticides as nontarget organism. Carbaryl is a carbamate pesticide and is commonly used against rice thrip pest in paddy fields. In the present work, cellular changes caused by exposure of the cyanobacterial biofertilizer namely Calothrix brevissima to carbaryl were studied with special reference to fatty acids, electrolyte leakage, sulfur metabolism, and osmolytes. To study the toxic effect of carbaryl, the test cyanobacterium was exposed to varying concentrations of pesticide (0, 10, 20, 30, and 40 mg L−1) for biochemical analyses. At 40 mg L−1 carbaryl, polyunsaturated fatty acids were reduced by 32 % and membrane leakage was increased by 27 % suggesting that free radical-mediated lipid peroxidation took place. The sulfur-containing metabolites namely cysteine, cystine, and methionine were increased by 79, 64, and 52 %, respectively. The enzymatic and nonenzymatic antioxidants namely glutathione S-transferase, glutathione reductase, reduced glutathione, and oxidized glutathione were increased to 56, 71, 72, and 60 %, respectively. Osmolytes that serve as stress enzyme protectors as well as nonenzymatic free radical scavenger were also increased, indicating their protective nature in context with carbaryl-induced stress. The respective increase in mannitol, trehalose, and glycogen were 158, 98, and 159 %. In C. brevissima, carbaryl-induced membrane leakage was counteracted by increasing enzymatic and nonenzymatic parameters that helped in scavenging free radicals.


Similar content being viewed by others
References
Anderson MI (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 20:418–425
Baisak A, Rana RD, Acharya PBB, Kar M (1994) Alteration in the activities of the active oxygen scavenging enzymes of wheat leaves subjected to water stress. Plant cell Physiol 35:489–495
Bandyopadhyay U, Das D, Banerjee RK (1999) Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci 77:658–666
Banerjee R, Becker D, Dickman M, Gladyshev V, Ragsdale S (eds) (2008) Redox biochemistry. Wiley Interscience, New Jersey
Bhadauriya P, Gupta R, Singh S, Bisen PS (2009) NaCl induced metabolic changes in the diazotrophic cyanobacterium Anabaena cylindrica. World J Microbiol Biotechnol 25:341–345
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Bohnert HJ, Jensen RG (1996) Strategies for engineering water-stress tolerance in plants. Trends Biotech 14:89–97
Booth E, Walker K (1997) The effectiveness of foliar glucosinolate content raised by sulfur application on disease control in oilseed rape. In: Cram WJ, De Kok LJ, Stulen I, Brunold C, Rennenberg H (eds) Sulfur metabolism in higher plants. Backhuys, Leiden, pp 327–329
Bowler CM, Montagu V, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Burcu S, Askim HS, Ismail T (2009) An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J Plant Growth Regul 28:12–20
Choudhary M, Jetely UK, Khan A, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline and malondialdehyde and superoxide dismutase activity in the cyanobacterium Spirulina platensis S-5. Ecotoxicol Environ Safety 66:20–49
Dubey SK, Rai LC (1989) Toxicity of chromium and tin to Anabaena doliolum interaction with sulfur-containing amino acids and thiols. Biol Metals 2:55–60
Fatma T, Khan MA, Choudhary M (2007) Impact of environmental pollution on cyanobacterial proline content. J Appl Phycol 19:625–629
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplast: a proposed role in ascorbic metabolism. Planta 133:21–25
Galhano V, Francisco P, Jose GL (2010) Bentazon triggers the promotion of oxidative damage in the Portuguese rice fields cyanobacterium Anabaena cylindrica: response of the antioxidant system. Environmental Toxicol 25:517–526
Galhano V, Santos H, Oliveira MM, Laranjo JG, Peixoto F (2011) Changes in fatty acid profile and antioxidant systems in a Nostoc muscorum strain exposed to the herbicide bentazon. Process Biochem 46:2152–2162
Gillian E, William H (2005) Determination of sugarcane deterioration at the factory: development of a rapid, easy and inexpensive enzymatic method to measure mannitol. Food Chem 98:366–372
Giordano M, Norici A, Ratti S, Raven JAR (2008) Role of sulfur for phytoplankton: acquisition, metabolism and ecology. In: Hell R, Leustek T, Knaff D, Dahl C (eds) Advances in photosynthesis research Vol. 27: sulfur metabolism in phototrophic organisms. Govindjee (series ed.). Springer, Berlin, pp 405–423
Gong M, Li YL, Chen SZ (1998) Abscisic acid-induced thermo tolerance in maize seedling is mediated by calcium and associated with antioxidant systems. J Plant Physiology 153:488–496
Grill D, Tausz M, De Kok LJ (eds) (2001) Significance of glutathione to plant adaptation to the environment. In: de Kok LJ, Stulen I (eds). Plant ecophysiology. Vol. 2. Kluwer Academic, Dordrecht
Gupta R, Bhadauriya P, Chauhan VS, Bisen PK (2008) Impact of UV-B radiation on thylakoid membrane and fatty acid profile of Spirulina platensis. Curr Microbiol 56:156–161
Habib K, Kumar S, Manikar N, Zutshi S, Fatma T (2011) Biochemical effect of carbaryl on oxidative stress, antioxidant enzymes and osmolytes of cyanobacterium Calothrix brevissima. Bull Environ Contam Toxicol 87:615–620
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hell R (1997) Molecular physiology of plant sulfur metabolism. Planta 202:138–148
Horn MJ, Jones DB, Blum AE (1946) Colorimetric determination of methionine in proteins and foods. J Biol Chem 166:313
Jingxian W, Ping X (2007) Antioxidant enzyme activities of Microcystis aeruginosa in response to nonylphenols and degradation of nonylphenols by M. aeruginosa. Environ Geochem Health 29:375–383
Kaushik BD (1998) Cyanobacteria response to salinity and amelioration technology. In: Kannaiyan S (ed) Rice management biotechnology. Associated, New Delhi, pp 323–329
Khan NA, Singh S, Umar S (eds) (2008) Sulfur assimilation and abiotic stress in plants. Springer, Berlin
Kumar N, Kumar RN, Bora A, Amb MK (2011) An evaluation of pesticide stress induced proteins in three cyanobacterial species Anabaena fertilissima, Aulosira fertilissima and Westiellopsis prolifica using SDS-PAGE. World Acad of Sci, Engg and Tech 75:2
Kumar S, Habib K, Fatma T (2008) Endosulfan induced biochemical changes in nitrogen-fixing cyanobacteria. Sci Total Environ 403:130–138
Latifi A, Ruiz M, Zhang CC (2009) Oxidative stress in cyanobacteria. FEMS Microbiol Rev 33:258–278
Leach SJ (1966) In: Alexander P, Lundgren HP (eds) A laboratory manual of analytical methods of protein chemistry, vol 4. Pergamon, Oxford, p 63
Leustek T, Saito K (1999) Sulfate transport and assimilation in plants. Plant Physiol 120:637–643
Liu X-J, Jiang Y, Chen F (2005) Fatty acid profile of the edible filamentous cyanobacterium Nostoc flagelliforme at different temperatures and developmental stages in liquid suspension culture. Process Biochem 40:371–377
Nadia B, Lee DH, Goldberg AL (2001) Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. The J of Bio Chem 276:24261–24267
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 499:249–279
Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647
Parrou JL, Francois J (1997) A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal Biochem 248:186–188
Prasad SM, Kumar D, Zeeshan M (2005) Growth, photosynthesis, active oxygen species and antioxidants responses of paddy field cyanobacterium Plectonema boryanum to endosulfan stress. J Gen Appl Microbiol 51:115–123
Rajendran UM, Kathirvel E, Anand N (2007) Desiccation-induced changes in antioxidant enzymes, fatty acids, and amino acids in the cyanobacterium Tolypothrix scytonemoides. World J Microbiol Biotechnol 23:251–257
Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opinion in Plant Bio 2:198–206
Ruiz JM, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Sakamoto T, Yoshida T, Arima H, Hatanaka Y, Takani Y, Tamaru Y (2009) Accumulation of trehalose in response to desiccation and salt stress in the terrestrial cyanobacterium Nostoc commune. Phycol Res 57:66–73
Sato N, Murata N (1988) Membrane lipids. Methods Enzymol 167:251–259
Schafer HJ, Greiner S, Rausch T, Haag-Derwer A (1997) In seedlings of the heavy metal accumulator Brassica juncea Cu2+ differentially affects transcript amounts for λ-glutamylcysteine synthetase (λ-ECS) and metallothionein (MT2). FEBS Lett 404:216–220
Shen BO, Richard GJ, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Singh SC, Sinha RP, Hader DP (2002) Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool 41:297–308
Stainer RY, Kunisawa R, Mandel M, Cohin-Bazire G (1971) Purification and properties of unicellular blue green algae (order Chroococcales). Bacteriol Rev 35:171–205
Stefels J (2000) Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J Sea Resear 43:183–197
Sunda W, Kieber DJ, Kiene RP, Huntsman S (2002) An antioxidant function for DMSP and DMS in marine algae. Nature 418:317–320
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol Environ Saf 71:1–15
Vass, Styring IS (1993) Characterization of chlorophyll triplet promoting states in photosystem II sequentially induced during photo inhibition. Biochemistry 32:3334–3341
Watanabe I, Roger PA (1984) Nitrogen fixation in wetland rice field. In: Subba Rao NS (ed) Current development in biological nitrogen fixation. Oxford and IBH, New Delhi, pp 237–276
Yap CY, Chen F (2001) Polyunsaturated fatty acids: biological significance, biosynthesis and production by microalgae and microalgae-like organisms. In: Chen F, Jiang Y (eds) Algae and their biotechnological potential. Kluwer Academic, Dordrecht, pp 1–32
Zeeshan M, Prasad SM (2009) Differential response of growth, photosynthesis, antioxidant enzymes and lipid peroxidation to UV-B radiation in three cyanobacteria. S Afr J Bot 75:466–474
Zutshi S, Meenakshi C, Naveen B, Abdin MZ, Fatma T (2008) Evaluation of antioxidant defense responses to lead stress in Hapalosiphon fontinnalis-339. J Phycol 44:889–896
Acknowledgments
We are thankful to DST and UGC, New Delhi, India for financial support, the National Centre for Utilization and Conservation of Blue Green Algae I.A.R.I., New Delhi for providing the C. brevissima culture, and Mr. Brahamdutt (Technical Assistant), I.A.R.I., New Delhi and Mr. Ajay Kumar (Senior Technical Assistant), J.N.U., New Delhi for providing necessary technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Habib, K., Manikar, N., Ansari, S. et al. Carbaryl stress induced cellular changes in Calothrix brevissima . Environ Sci Pollut Res 20, 862–871 (2013). https://doi.org/10.1007/s11356-012-1217-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1217-1