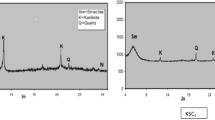
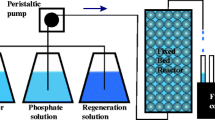
Abstract
The purpose of this research is to study the effect of a new method of adsorption using membrane filtration to determine the maximum amount of lead adsorbed by clay and investigate the behavior of the clay after adsorption of the said metal. Treatment of wastewater contaminated with heavy metals depends on the characteristics of the effluent, the amount of final discharge, the cost of treatment, and the compatibility of the treatment process. The process of adsorption of heavy metals by clays may be a simple, selective, and economically viable alternative to the conventional physical–chemical treatment. This is justified by the importance of the surface developed by this material, the presence of negative charges on the said surface, the possibility of ion exchange taking place, and its wide availability in nature. The removal of lead from wastewater was studied by using the adsorption technique and using clay as the adsorbent. A method was optimized for adsorption through a membrane approaching natural adsorption. This new method is simple, selective, and the lead adsorption time is about 3 days. The various properties of clay were determined. It was observed that the cation exchange capacity of the clay was 56 meq/100 g of hydrated clay for the raw sample and 82 meq/100 g for the purified sample. The total surface area determined by the methylene blue method was equal to 556 and 783 m²/g for the raw and purified samples, respectively. The adsorption kinetics depends on several parameters. The Pb(II) clay, obeys the Langmuir, Freundlich, and the Elovich adsorption isotherms with high regression coefficients. The use of this adsorbent notably decreases the cost of treatment. It was concluded that clay shows a strong adsorption capacity on Pb(II), the maximum interaction occurring with purified clay treated at high concentration of lead. It is proposed that this adsorption through a membrane be extended for the treatment of effluents containing other metals.








Similar content being viewed by others
References
Alloway B (1990) Heavy metals in soils. Wiley. New York. Atténuation des métaux à l'aval de sites de stockage de déchets—Synthèse bibliographique66 BRGM/RP-54417-FR - Rapport final.
Audry S (2003) Bilan géochimique du transport des éléments métalliques dans le système fluvial anthropisé Lot-Garonne-Gironde, Université de Bordeaux I, 415 pp.
Ayari F, Srasra E, Trabelsi-Ayadi M (2007) Retention of lead from an aqueous solution by use of bentonite as adsorbent for reducing leaching from industrial effluents. Desalination 206:270–277
Ballet GT, Gzara L, Hafiane A, Dhahbi M (2004) Transport coefficients and cadmium salt rejection in nanofiltration membrane. Desalination 167:369–376
Barbier F, Duc G, Petit-Ramel M (2000) Adsorption of lead and cadmium ions from aqueous solution on the montmorillonite/water interface. Colloids Surf A 166:153–159
Bazrafshan E, Mahvi AH, Nasseri S, Mesdaghinia AR, Vaezi F, Nazmara S (2006) Removal of cadmium from industrial effluents by electrocoagulation process using iron electrodes, Iran. J Environ Health Sci Eng 3(4):261–266
Bradbury MH, Baeyens B (1997) A mechanistic description of Ni and Zn sorption on Na-montmorillonite Part II: modelling. J Contam Hydrol 27:223–248
Chen C, Coleman M, Katz L (2006) Bridging the gap between macroscopic and spectroscopic studies of metal ion sorption at the oxide/water interface: Sr(II), Co(II), and Pb(II) sorption to quartz. Environ Sci Technol 40:142–148
Christ I, Kretzschmar R (1999) Competitive sorption of copper and lead at the oxide–water interface: implications for surface site density. Geochim Cosmochim Acta 63:2929–2938
Edzwald JK (2010) Dissolved air flotation and me. Water Res 44:2077–2106
Eloussaief M, Jarraya I, Benzina M (2009) Adsorption of copper ions on two clays from Tunisia: pH and temperature effects. Appl Clay Sci 46:409–413
Erdem E, Karapinar N, Donat R (2008) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Fan Q, Li Z, Zhao H, Jia Z, Xu J, Wu W (2009) Adsorption of Pb(II) on palygorskite from aqueous solution: effects of pH, ionic strength and temperature. Appl Clay Sci 45:111–116
Ghorbel-Abid I, Jrad A, Nahdi K, Trabelsi-Ayadi M (2009) Sorption of chromium (III) from aqueous solution as bentonitic clay. Desalination 246:595–604
Ghorbel-Abid I, Galai K, Trabelsi-Ayadi M (2010) Retention of chromium (III) and cadmium(II) from aqueous solution by illitic clay as a low-cost adsorbent. Desalination 256:190–195
Gua X, Evans Les J, Barabash SJ (2010) Modeling the adsorption of Cd (II), Cu (II), Ni (II), Pb (II) and Zn (II) onto montmorillonite. Geochim Cosmochim Acta 74:5718–5728
Guo L, Zhang SF, Ju BZ, Yang JZ (2006) Study on adsorption of Cu(II) by water-insoluble starch phosphate carbamate. Carbohydr Polym 63:487–492
Gupta VK (2005) Adsorption of As (III) from aqueous solutions by iron oxide-coated sand. J Colloid Interface Sci 288:55–60
Gupta VK, Bhattacharyya KG (2006) Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium derivatives. J Hazard Mater 128:247–257
Gupta VK, Imran A (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271(2):321–328
Gupta VK, Mohan D (1999) Removal of chromium(VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Gupta VK, Rastogi A (2008a) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2008b) Biosorption of lead from aqueous solutions by green algae Spirogyra species: equilibrium and adsorption kinetics. J Hazard Mater 152(1):407–414
Gupta VK, Srivastava SK, Mohan D, Sharma S (1997) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manag 17:517–522
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mudan aluminium industry waste. Water Res 35:1125–1134
Gupta VK, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45(4):1446–1453
Gupta VK, Jain R, Varshney S (2007) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Carrott PJM, Robeiro Carrott MML, Suhas (2009a) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39(10):783–842
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas (2009b) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Hang PT, Brindley GW (1970) Methylene blue adsorption of clay minerals. Clay Clay Miner 18:203–212
Ho YS, Mckay G (1999) The sorption of lead ions on peat. Water Res 33:578–584
Hyun SP, Cho YH, Hahn PS, Kim SJ (2001) Sorption mechanism of U(VI) on a reference montmorillonite: binding to the internal and external surfaces. J Radioanal Nucl Chem 250:55–62
Ikhsan J, Wells JD, Johnson BB, Angove MJ (2005) Surface complexation modeling of the sorption of Zn(II) by montmorillonite. Colloids Surf A 252:33–41
Imran A, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1(6):2661–2667
Karakisla M (2003) The adsorption of Cu(II) ion from aqueous solution upon acrylic acid grafted poly(ethylene terephthalate) fibers. J Appl Polymer Sci 87:1216–1220
Khalfaoui M, Knani S, Hachicha MA, Ben Lamine A (2003) New theoretical expressions for the adsorption type isotherms classified by BET based on statistical physics treatment. J Colloid Interface Sci 263:350–356
Kipling JJ, Wilson RP (1960) Adsorption of methylene blue in the determination of surface areas. J Appl Chem 10(3):109–113
Kul AR, Koyuncu H (2010) Adsorption of Pb (II) ions from aqueous solution by native and activated bentonite: kinetic, equilibrium and thermodynamic study. J Hazard Mater 179:332–339
Lin S, Juang R (2002) Heavy metal removal from water by sorption using surfactant modified montmorillonite. J Hazard Mater 92:315–326
Lutzenkirchen J (1997) Ionic strength effects on cation sorption to oxides: macroscopic observations and their significance in microscopic interpretation. J Colloid Interface Sci 195:149–155
M'bodj O, Kbir-Ariguib N, Trabelsi-Ayadi M, Magnin A (2004) Plastic and elastic properties of the systems interstratified clay–water–electrolyte–xanthan. J Colloid Interface Sci 273:675–684
Merzouk B, Gourich B, Sekki A, Madani K, Chibane M (2009) Removal turbidity and separation of heavy metals using electrocoagulation–electroflotation technique. J Hazard Mater 164:215–222
Mhamdi M, Gasmi N, Elaloui E, Kbir-Ariguib N,Trabelsi-Ayadi M (2010a). Study of the mechanical properties of clay containing smectite and carbonate. IOP Conf. Series: Materials Science and Engineering 13–012027.
Mhamdi M, Gasmi N, Elaloui E, Kbir-Ariguib N, Trabelsi-Ayadi M (2010b) Purification and characterization of smectite clay taken from Gafsa, Tunisia: progressive elimination of carbonates. IOP Conf. Series: Materials Science and Engineering 13–012030.
Mokaddem H, Sadaoui Z, Boukhelata N, Azouaou N, Kaci Y (2009) Removal of cadmium aqueous solution by polysaccharide produced from Paenibacillus polymyxa. J Hazard Mater 172:1150–1155
Mouni L, Merabet D, Robert D, Bouzaza A (2009) Batch studies for the investigation of the sorption of the heavy metals Pb2+ and Zn2+ onto Amizour soil (Algeria). Geoderma 154:30–35
Mushrif SH, Rey AD (2009) An integrated model for adsorption induced strain in microporous solids. Chem Eng Sci 64:4744–4753
Pabalan RT, Turner DR (1997) Uranium (6+) sorption on montmorillonite: experimental and surface complexation modeling study. Aquat Geochem 2:203–226
Potgieter JH, Potgieter-Vermaak SS, Kalibantonga PD (2006) Heavy metals removal from solution by palygorskite clay. Miner Eng 19:463–470
Qiaohui F, Zhan L, Haogui Z, Zehong J, Junzheng X, Wangsuo W (2009) Adsorption of Pb(II) on palygorskite from aqueous solution: effects of pH, ionic strength and temperature. Appl Clay Sci 45:111–116
Rabie A, Usman A (2008) The relative adsorption selective of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley, Egypt. Geoderma 144:334–343
Sdiri A, Higashi T, Chaabouni R, Jamoussi F (2012) Competitive removal of heavy metals from aqueous solutions by montmorillonitic and calcareous clays. Water Air Soil Pollut 223:1191–1204
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Environ Eng 123(5):461–468
Stader M, Schindler PW (1993) Modeling of H+ and Cu2+ adsorption on calcium-montmorillonite. Clays Clay Miner 41:288–296
Stumm W (1992) Chemistry of the solid–water interface: processes at the mineral–water and particle–water interface in natural systems. Wiley, New York
Zachara JM, Smith SC (1994) Edge complexation reactions of cadmium on specimen and soil-derived smectite. Soil Sci Soc Am J 58:762–769
Acknowledgments
We greatly acknowledge the financial support of the Ministry of Higher Education and Scientific Research of Tunisia. We extend our thanks to Mr Naceur Belgacem, Professor of Grenoble INP-Pagora LGP2 (UMR 5518) CNRS for his help. The authors would like to thank both the reviewers for helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Mhamdi, M., Galai, H., Mnasri, N. et al. Adsorption of lead onto smectite from aqueous solution. Environ Sci Pollut Res 20, 1686–1697 (2013). https://doi.org/10.1007/s11356-012-1015-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1015-9