Abstract
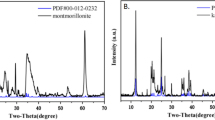
Sorption interactions with montmorillonite and other clay minerals in soils, sediments, and rocks are potentially important mechanisms for attenuating the mobility of U(6+) and other radionuclides through the subsurface environment. Batch experiments were conducted (in equilibrium with atmospheric % MathType!MTEF!2!1!+-% feaafeart1ev1aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn% hiov2DGi1BTfMBaeXafv3ySLgzGmvETj2BSbqefm0B1jxALjhiov2D% aebbfv3ySLgzGueE0jxyaibaiiYdd9qrFfea0dXdf9vqai-hEir8Ve% ea0de9qq-hbrpepeea0db9q8as0-LqLs-Jirpepeea0-as0Fb9pgea% 0lrP0xe9Fve9Fve9qapdbaqaaeGacaGaaiaabeqaamaabaabcaGcba% acbiGaiWiG-bfadaWgaaWcbaacbaGaa43qaiaa+9eadaWgaaqaaiaa% +jdaaWqabaaaleqaaaaa!400D!\[P_{CO_2 } \])to determine the effects of varying pH (2 to 9), solid-mass to solution-volume ratio (M/V = 0.028 to 3.2 g/L), and solution concentration (2 × 10−7 and 2 × 10−6 M 233U) on U(6+) sorption on SAz-1 montmorillonite. The study focused on U(6+) surface complexation on hydroxylated edge sites as the sorption mechanism of interest because it is expected to be the predominant sorption mechanism at pHs typical of natural waters (pH ≈6 to ≈9). Thus, the experiments were conducted with a 0.1 M NaNO3 matrix to suppress ion-exchange between U(6+) in solution and interlayer cations. The results show that U(6+) sorption on montmorillonite is a strong function of pH, reaching a maximum at near-neutral pH (≈6 to ≈6.5) and decreasing sharply towards more acidic or more alkaline conditions. A comparison of the pH-dependence of U(6+) sorption with that of U(6+) aqueous speciation indicates a close correspondence between U(6+) sorption and the predominance field of U(6+)-hydroxy complexes. At high pH, sorption is inhibited due to formation of aqueous U(6+)-carbonate complexes. At low pH, the low sorption values indicate that the 0.1 M NaNO3 matrix was effective in suppressing ion-exchange between the uranyl (UO2 2+) species and interlayer cations in montmorillonite. At pH and carbonate concentrations typical of natural waters, sorption of U(6+) on montmorillonite can vary by four orders of magnitude and can become negligible at high pH.
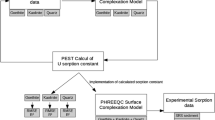
The experimental results were used to develop a thermodynamic model based on a surface complexation approach to permit predictions of U(6+) sorption at differing physicochemical conditions. A Diffuse-Layer model (DLM) assuming aluminol (>AlOHℴ) and silanol (>SiOHℴ) edge sites and two U(6+) surface complexation reactions per site effectively simulates the complex sorption behavior observed in the U(6+)-H2O-CO2-montmorillonite system at an ionic strength of 0.1 M and pH > 3.5. A comparison of model predictions with data from this study and from published literature shows good agreement and suggests that surface complexation models based on parameters derived from a limited set of data could be useful in extrapolating radionuclide sorption over a range of geochemical conditions. Such an approach could be used to support transport modeling by providing a better alternative to the use of constant K d s in transport calculations.
Similar content being viewed by others
References
Akcay H. and Kurtulmus F. (1995) Study of uranium sorption and desorption on some Turkish clays. J. Radioanal. Nucl. Chem. Lett. 200, 529–544.
Allard B., Beall G. W. and Krajewski T. (1980) The sorption of actinides in igneous rocks. Nucl. Technol. 49, 474–480.
Allison J.D., Brown D.S. and Novo-Gradac K.J. (1991) MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0 User's manual. EPA/600/3–91/021. Environmental Protection Agency, Athens, GA.
Ames L. L., McGarrah J. E. and Walker B. A. (1983) Sorption of trace constituents from aqueous solutions onto secondary minerals. I. Uranium. Clays Clay Miner. 31, 321–334.
Benjamin M. M. and Leckie J. O. (1981) Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Colloid Interf. Sci. 27, 305–318.
Bertetti F. P., Pabalan R. T., Turner D. T. and Almendarez M. G. (in prep.) Experimental and modeling study of uranium(6+) sorption on quartz.
Bertetti F. P., Pabalan R. T. and Almendarez M. G. (accepted for publication) Studies of neptunium (V) sorption on quartz, clinoptilolite, montmorillonite, and α-alumina. (ed. E. Jenne), Sorption of Metals by Earth Materials, Academic Press, New York.
Borovec Z. (1981) The adsorption of uranyl species by fine clay. Chem. Geol. 32, 45–58.
Bradbury M. H. and Baeyens B. (1993) A general application of surface complexation to modeling radionuclide sorption in natural systems. J. Colloid Interf. Sci. 158, 364–371.
Chisholm-Brause C., Conradson S. D., Buscher C. T., Eller P. G., and Morris D. E. (1994) Speciation of uranyl sorbed at multiple binding sites on montmorillonite. Geochim. Cosmochim. Acta 58, 3625–3631.
Choppin G. R., and Mathur J. N. (1991) Hydrolysis of actinyl(VI) cations. Radiochim. Acta 52/53, 25–28.
Davis J. A. and Leckie J. O. (1978) Surface ionization and complexation at the oxide/water interface II. Surface properties of amorphous iron oxyhydroxide and adsorption of metal ions. J. Colloid Interf. Sci. 67, 90–107.
Davis J. A. and Kent D. B. (1990) Surface complexation modeling in aqueous geochemistry. In Reviews in Mineralogy: Volume 23. Mineral-Water Interface Geochemistry (eds M. F. Hochella, Jr. and A. F. White), pp. 177–260. Mineralogical Society of America, Washington, D.C.
Degueldre C., Ulrich J. J. and Silby H. (1994) Sorption of241Am onto montmorillonite, illite and hematite colloids. Radiochim. Acta 65, 173–179.
Dozol M. and Hagemann R. (1993) Radionuclide migration in groundwaters — Review of the behaviour of actinides. Pure Appl. Chem. 65, 1081–1102.
Dzombak D. A. and Morel F. M. M. (1990) Surface Complexation Modeling: Hydrous Ferric Oxide. John Wiley and Sons, New York.
Fuger, J. (1992) Thermodynamic properties of actinide aqueous species relevant to geochemical problems. Radiochim. Acta 58/59, 81–91.
Grauer R. (1994) Bentonite as a backfill material in a high-level waste repository. MRS Bull. 19, 43–46.
Hayes K. F., Redden G. G., Ela W. and Leckie J. O. (1991) Surface complexation models: An evaluation of model parameter estimation using FITEQL and oxide mineral titration data. J. Colloid Interf. Sci. 142, 448–469.
Hsi C-K. D. and Langmuir D. (1985) Adsorption of uranyl onto ferric oxyhydroxides: Application of the surface complexation site-binding model. Geochim. Cosmochim. Acta 49, 1931–1941.
LaFlamme B. D. and Murray J. W. (1987)Solid/Solution Interaction: The effect of carbonate alkalinity on adsorbed thorium. Geochim. Cosmochim. Acta 51, 243–250.
Marmier N., Dumonceau J., Chupeau J., and Fromage F. (1995) Modeling of Yb(III) sorption on kaolinite by using single oxide surface complexation models. In Scientific Basis for Nuclear Waste Management XVIII (eds. T. Murakami and R. C. Ewing), Materials Research Society Symposium Proceedings 353, Materials Research Society, Pittsburgh, PA, pp. 1085–1092.
McKinley J. P., Zachara J. M., Smith S. C. and Turner G. D. (1995) The influence of hydrolysis and multiple site-binding reactions on adsorption of U(VI) to montmorillonite. Clays Clay Miner. 43, 586–598.
Morris D. E., Chisholm-Brause C. J., Barr M. E., Conradson S. D. and Eller P. G. (1994) Optical spectroscopic studies of the sorption of UO2 2+ species on a reference smectite. Geochim. Cosmochim. Acta 58, 3613–3623.
Pabalan R. T., Turner D. R., Bertetti F. P., and Prikryl J. P. (accepted for publication) Uranium(VI) sorption onto selected mineral surfaces: Key geochemical parameters. In Sorption of Metals by Earth Materials (ed. E. Jenne), Academic Press, New York.
Pabalan R. T., Turner D. R. and Bertetti F. P. (1994) Sorption modeling for HLW Performance Assessment. In NRC High-Level Radioactive Waste Research at CNWRA January–June, 1994 (ed. B. Sagar), CNWRA 94–01S, Center for Nuclear Waste Regulatory Analyses, San Antonio, TX.
Pabalan R. T., Prikryl J. D., Muller P. M. and Dietrich T. B. (1993) Experimental study of uranium(6+) sorption on the zeolite mineral clinoptilolite. In Scientific Basis for Nuclear Waste Management XVI (eds. C. G. Interrante, and R. T. Pabalan), Materials Research Society Symposium Proceedings 294, Pittsburgh, PA, pp. 777–782.
Payne T. E., Sekine K. Davis J. A. and Waite T. D. (1992) Modeling of radionuclide sorption processes in the weathered zone of the Koongarra ore body. In Alligator Rivers Analogue Project Annual Report, 1990–1991 (ed. P. Duerden), Australian Nuclear Science and Technology Organization (ANSTO), pp. 57–85.
Prikryl J. D., Pabalan R. T., Turner D. R. and Leslie B. W. (1994) Uranium sorption on α-alumina: Effects of pH and surface-area/solution-volume ratio. Radiochim. Acta 66/67, 291–296.
Rai D., Zachara J. M., Eary L. E., Ainsworth C. C., Amonette J. E., Cowan C. E., Szelmeczka R.W., Resch C. R., Schmidt R. L., Girvin D. C. and Smith S. C. (1988) Chromium Reactions in Geologic Materials. EPRI-EA-5741, Electric Power Research Institute, Palo Alto, CA.
Sikalidis C. A., Alexiades C. and Misaelides P. (1989) Adsorption of uranium and thorium from aqueous solutions by the clay minerals montmorillonite and vermiculite. Toxicol. Environ. Chem. 20/21, 175–180.
Tripathi V. S. (1984) Uranium(VI) Transport Modeling. Geochemical Data and Submodels. Unpublished Ph.D. Thesis. Stanford University, Stanford, CA.
Tsunashima A., Brindley G. W. and Bastovano M. (1981) Adsorption of uranium from solutions by montmorillonite; compositions and properties of uranyl montmorillonites. Clays Clay Miner. 29, 10–16.
Turner D. (1995) A Uniform Approach to Surface Complexation Modeling of Radionuclide Sorption. CNWRA 95–001. Center for Nuclear Waste Regulatory Analyses, San Antonio, TX.
Turner D. R. and Sassman S. A. (1996) Approaches to sorption modeling for high-level waste performance assessment. J. Contam. Hydrol. 21, 311–332.
van Geen A., Robertson A. P. and Leckie J. O. (1994) Complexation of carbonate species atthe goethite surface: Implications for adsorption of metal ions in natural waters. Geochim. Cosmochim. Acta 58, 2073–2086.
Waite T. D., Davis J. A., Payne T. E., Waychunas G. A. and Xu N. (1994) Uranium(VI) adsorption to ferrihydrite: Application of a surface complexation model. Geochim. Cosmochim. Acta 58, 5465–5478.
Wanner H. and Forest I., eds. (1992) Chemical Thermodynamics of Uranium. North-Holland, Amsterdam.
Wanner H., Albinsson Y., Karnl O., Wieland E., Wersin P. and Charlet L. (1994) The acid/base chemistry of montmorillonite. Radiochim. Acta 66/67, 733–738.
Westall J. C. (1982a) FITEQL: A Computer Program for Determination of Chemical Equilibrium Constants From Experimental Data, Version 1.2. Rpt. 82–01. Department of Chemistry, Oregon State University, Corvallis, OR.
Westall J. C. (1982b) FITEQL: A Computer Program for Determination of Chemical Equilibrium Constants From Experimental Data, Version 2.0. Rpt. 82–02. Department of Chemistry, Oregon State University, Corvallis, OR.
Westall J. C. and Hohl H. (1980) A comparison of electrostatic models for the oxide/solution interface. Adv. Colloid Interf. Sci. 12, 265–294.
White G. N. and Zelazny L. W. (1988) Analysis and implications of the edge structure of dioctahedral phyllosilicates. Clays Clay Miner. 36, 141–146.
Wolery T. J. (1992) EQ3NR, A Computer Program for Geochemical Aqueous Speciation-Solubility Calculations: Theoretical Manual, User's Guide, and Related Documentation (Version 7.0). UCRL-MA-110662- Pt.3. Lawrence Livermore National Laboratory. Livermore, CA.
Zachara J. M., and McKinley J. P. (1993) Influence of hydrolysis on the sorption of metal cations by smectites: Importance of edge coordination reactions. Aquatic Sci. 55, 250–261.
Zachara J. M. and Smith S. C. (1994) Edge complexation reactions of cadmium on specimen and soil-derived smectite. Soil Sci. Soc. Amer. J. 58, 762–769.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pabalan, R.T., Turner, D.R. Uranium(6+) sorption on montmorillonite: Experimental and surface complexation modeling study. Aquat Geochem 2, 203–226 (1997). https://doi.org/10.1007/BF00119855
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00119855