Abstract
Background, aim, and scope
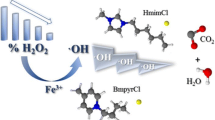
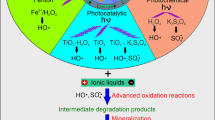
Ionic liquids are regarded as essentially “green” chemicals because of their insignificant vapor pressure and, hence, are a good alternative to the emissions of toxic conventional volatile solvents. Not only because of their attractive industrial applications, but also due to their very high stability, ionic liquids could soon become persistent contaminants of technological wastewaters and, moreover, break through into natural waters following classical treatment systems. The removal of harmful organic pollutants has forced the development of new methodologies known as advanced oxidation processes (AOPs). Among them, the Fenton and Fenton-like reactions are usually modified by the use of a higher hydrogen peroxide concentration and through different catalysts. The aim of this study was to assess the effect of hydrogen peroxide concentration on degradation rates in a Fenton-like system of alkylimidazolium ionic liquids with alkyl chains of varying length and 3-methyl-N-butylpyridinium chloride.
Materials and methods
The ionic liquids were oxidized in dilute aqueous solution in the presence of two different concentrations of hydrogen peroxide. All reactions were performed in the dark to prevent photoreduction of Fe(III). The concentrations of ionic liquids during the process were monitored with high-performance liquid chromatography. Preliminary degradation pathways were studied with the aid of 1H NMR.
Results
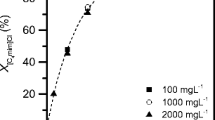
Degradation of ionic liquids in this system was quite effective. Increasing the H2O2 concentration from 100 to 400 mM improved ionic liquid degradation from 57–84% to 87–100% after 60 min reaction time. Resistance to degradation was weaker, the shorter the alkyl chain.
Discussion
The compound omimCl was more resistant to oxidation then other compounds, which suggests that the oxidation rates of imidazolium ionic liquids by OH· are structure-dependent and are correlated with the n-alkyl chain length substituted at the N-1-position. The level of degradation was dependent on the type of head group. Replacing the imidazolium head group with pyridinium increased resistance to degradation. Nonetheless, lengthening the alkyl chain from four to eight carbons lowered the rate of ionic liquid degradation to a greater extent than changing the head group from imidazolium to pyridinium. 1H-NMR spectra show, in the first stage of degradation, that it is likely that radical attack is nonspecific, with any one of the carbon atoms in the ring and the n-alkyl chain being susceptible to attack.
Conclusions
The proposed method has proven to be an efficient and reliable method for the degradation of imidazolium ionic liquids by a Fenton-like reagent deteriorated with lengthening n-alkyl substituents and by replacing the imidazolium head group with pyridinium. The enhanced resistance of 1-butyl-3-methylpyridinium chloride when the resistance of imidazolium ionic liquids decreases with increasing H2O2 concentration is probably indicative of a change in the degradation mechanism in a vigorous Fenton-like system. H-NMR spectra showed, in the first stage of degradation, that radical attack is nonspecific, with any one of the carbon atoms in the ring and the n-alkyl chain being susceptible to attack.
Recommendations and perspectives
Since ionic liquids are now one of the most promising alternative chemicals of the future, the degradation and waste management studies should be integrated into a general development research of these chemicals. In the case of imidazolium and pyridinium ionic liquids that are known to be resistant to bio- or thermal degradation, studies in the field of AOPs should assist the future structural design as well as tailor the technological process of these chemicals



Similar content being viewed by others
References
Blake DM (1997) Bibliography of work on the photocatalytic removal of hazardous compounds from water and air. National renewable Energy laboratory report NREL/TP-430-22197
Eisenberg GM (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem 15:327–328
Gathergood N, Garcia MT, Scammells PJ (2004) Biodegradable ionic liquids: Part I. Concept, preliminary targets and evaluation. Green Chem 3:166–175
Holbrey JD, Seddon KR (1990) Ionic liquids. Clean Prod Proc 1:223–226
Jastorff B, Mölter K, Behrend P, Bottin-Weber U, Filser J, Heimers A, Ondruschka B, Ranke J, Schaefer M, Schröder H, Stark A, Stepnowski P, Stock F, Störmann R, Stolte S, Welz-Biermann U, Ziegert S, Thöming J (2005) Progress in evaluation of risk potential of ionic liquids—basis for an eco-design of sustainable products. Green Chem 7:362–372
Khodadoust AP, Chandrasekaran S, Dionysiou DD (2006) Preliminary assessment of imidazolium-based room-temperature ionic liquids for extraction of organic contaminants from soils. Environ Sci Technol 40:2339–2345
Kowalska S, Buszewski B, Stepnowski P (2006) The influence of stationary phase properties on ionic liquid cations separation in RP-HPLC. J Sep Sci 29:1116–1125
Montgomery HAC, Dymock JF, Thom NS (1962) The rapid colorimetric determination of organic acids and their salts in sewage-sludge liquor. The Analysts 87:949–955
Rogers RD, Seddon K (2002) Ionic liquids: Industrial applications for Green Chemistry. American Chemical Society ACS Ser. 818. Oxford University Press, Washington DC
Siedlecka E, Stepnowski P (2006a) Decomposition rates of methyl tert butyl ether and its by-products by the Fenton system in saline wastewaters. Sep Purif Technol 52:317–324
Siedlecka E, Stepnowski P (2006b) Treatment of oily port wastewater effluents using the UV/H2O2 photodecomposition system. Water Environ Res 78(8):852–856
Siedlecka EM, Mrozik W, Kaczyński Z, Stepnowski P (2008) Degradation of 1-butyl-3-methylimidazolium chloride ionic liquid in a Fenton-like system. J Hazard Mater 154:893–900
Stepnowski P (2005) Solid phase extraction of ionic liquids from environmental aqueous samples. Anal Bioanal Chem 381(1):189–193
Stepnowski P, Mrozik W (2005) Analysis of selected ionic liquid cations by ion exchange chromatography and reversed-phase high performance liquid chromatography. J Sep Sci 28:149–154
Stepnowski P, Storoniak P (2005) Lipophilicity and metabolic route prediction of imidazolium ionic liquids. Environ Sci Pollut Res 12(4):199–204
Stepnowski P, Zaleska A (2005) Comparison of different advanced oxidation processes for the degradation of room temperature ionic liquids. J Photochem Photobiol A:Chem 170:45–50
Stepnowski P, Müller A, Behrend P, Ranke J, Hoffmann J, Jastorff B (2003) Reversed-phase liquid chromatography of selected room-temperature ionic cations. J Chrom A 993:173–178
Stepnowski P, Nichthauser J, Mrozik W, Buszewski B (2006) Usefulness of π...π aromatic interactions in the selective separation and analysis of imidazolium and pyridinium ionic liquid cations. Anal Bioanal Chem 385:1483–1491
Walling C (1975) Fenton’s reagent revisited. Acc Chem Res 8:125–131
Wasserscheid P, Welton T (2002) Ionic liquids in synthesis. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Wu K, Xie Y, Zhao J, Hidaka H (1999) Photo-Fenton degradation of dye under visible light irradiation. J Mol Cat A:Chem 144:77–84
Yang Q, Dionysiou DD (2004) Photolytic degradation of chlorinated phenols in room temperature ionic liquids. J Photochem Photobiol A 165:229–240
Acknowledgment
Financial support was provided by the Polish Ministry of Research and Higher Education under grants: BW 8000-5-0135-8, DS 8270-4-0093-8, 2P04G 083 29, 2P04G 118 29 and DS 8200-4-0085-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siedlecka, E.M., Stepnowski, P. The effect of alkyl chain length on the degradation of alkylimidazolium- and pyridinium-type ionic liquids in a Fenton-like system. Environ Sci Pollut Res 16, 453–458 (2009). https://doi.org/10.1007/s11356-008-0058-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-008-0058-4