Abstract
Purpose
Recently brown adipose tissue (BAT) activation has been proposed to have a possible role in breast cancer. The aim of this study was to evaluate BAT activation in patients with breast cancer and its relationship with molecular characteristics of tumor.
Procedures
The study group comprised 79 patients with histologically proven ductal breast carcinoma (51 ± 13 years). Data on distribution, intensity (SUVmax), and total metabolic activity (TMA) of BAT were obtained from [18F] FDG-PET/CT. Clinical and biochemical data were obtained from the database.
Results
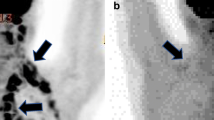
BAT activation was present in 12 of the 79 patients (15.2 %). Patients with BAT activation were younger and had a lower body mass index (BMI) (p < 0.05 and p < 0.0005, respectively) and showed less frequently metastasis (p < 0.05). No significant differences were found in estrogen receptor (ER), progesterone receptor (PgR), Ki67, grade, and in molecular subtypes. In patients younger than 55 years and with a BMI < 26, no significant differences were observed between patients with and without BAT activation. In the 12 patients with BAT activation, a significant inverse correlation was observed between TMA and BMI (r = − 0.64, p < 0.05). TMA and SUVmax were higher in grade 2 than in grade 3 patients. No significant differences were found in both TMA and SUVmax between patients with and without lymph node metastases. A significant difference in both TMA and SUVmax was observed among different molecular types, with luminal B patients showing higher values.
Conclusions
In conclusion, the present study suggests a relation between BAT activation and positive known prognostic factor in breast cancer, such as intermediate tumor grade and luminal B cancer type.
Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, Bran B (2014) Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging 41:776–791
Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK (2002) Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 29:1393–1398
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
Pasanisi F, Pace L, Fonti R, Marra M, Sgambati D, De Caprio C, De Filippo E, Vaccaro A, Salvatore M, Contaldo F (2013) Evidence of brown fat activity in constitutional leanness. J Clin Endocrinol Metab 98:1214–1218
Berstein LM (2012) Cancer and heterogeneity of obesity: a potential contribution of brown fat. Future Oncol 8:1537–1548
Jones LP, Buelto D, Tago E, Owusu-Boaitey KE (2011) Abnormal mammary adipose tissue environment of Brca1 mutant mice show a persistent deposition of highly vascularized multilocular adipocytes. J Cancer Sci Ther 8(Suppl 2). https://doi.org/10.4172/1948-5956.s2-004
Huang YC, Chen TB, Hsu CC, Li SH, Wang PW, Lee BF, Kuo CY, Chiu NT (2011) The relationship between brown adipose tissue activity and neoplastic status: an (18)F-FDG PET/CT study in the tropics. Lipids Health Dis 10:238–244
Cao Q, Hersl J, La H, Smith M, Jenkins J, Goloubeva O, Dilsizian V, Tkaczuk K, Chen W, Jones L (2014) A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 14:126–131
Fujii T, Yajima R, Tatsuki H, Oosone K, Kuwano H (2017) Implication of atypical supraclavicular F18-fluorodeoxyglucose uptake in patients with breast cancer: association between brown adipose tissue and breast cancer. Oncol Lett 14(6):7025–7030
Bos SA, Gill CM, Martinez-Salazar EL, Torriani M, Bredella MA (2019) Preliminary investigation of brown adipose tissue assessed by PET/CT and cancer activity. Skelet Radiol 48(3):413–419
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24:2206–2223
Pace L, Nicolai E, D’Amico D, Ibello F, Della Morte AM, Salvatore B, Pizzuti LM, Salvatore M, Soricelli A (2011) Determinants of physiologic 18F-FDG uptake in brown adipose tissue in sequential PET/CT examinations. Mol Imaging Biol 13:1029–1035
Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji Bm Chatal JF, Resche I, Campone M (2006) Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging 33:785–791
Dobert N, Menzel C, Hamscho N, Wordehoff N, Kranert WT, Grünwald F (2004) Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Q J Nucl Med Mol Imaging 48:33–38
Howlader N, Cronin KA, Kurian AW, Andridge R (2018) Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev 27(6):619–626
Lim S, Hosaka K, Nakamura M, Cao Y (2016) Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget 7(25):38282–38291
Chan XHD, Balasundaram G, Attia ABE, Goggi JL, Ramasamy B, Han W et al (2018) Multimodal imaging approach to monitor browning of adipose tissue in vivo. J Lipid Res 59:1071–1078
Wang H, Willershäuser M, Karlas A, Gorpas D, Reber J, Ntziachristos V et al (2019) A dual Ucp1 reporter mouse model for imaging and quantitation of brown and brite fat recruitment. Mol Metab 20:14–27
Lim S, Honek J, Xue Y, Seki T, Cao Z, Andersson P, Yang X, Hosaka K, Cao Y (2012) Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat Protoc 7:606–615
Gao D, Vahdat LT, Wong S, Chang JC, Mittal V (2012) Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res 72:4883–4889
Gennaro N, Pepe G, Antunovic L (2019) 18F-FDG uptake of brown fat and cancer: casualty or causality? Eur J Nucl Med Mol Imaging 46:1395–1396
Author information
Authors and Affiliations
Contributions
LP conceived the study, performed the statistical analysis, and wrote the manuscript. EN participated in its design and coordination and helped to draft the manuscript. LB acquired the scans and elaborated them. NG acquired the scans and elaborated them. AS participated in the study design and coordination. MS was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the Institutional Ethics Committee (Prot 2-11, IRCCS Fondazione SDN).
Informed Consent
For this type of study, retrospective, formal consent is not required.
Consent for Publication
The manuscript does not contain individual person’s data in any form.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pace, L., Nicolai, E., Basso, L. et al. Brown Adipose Tissue in Breast Cancer Evaluated by [18F] FDG-PET/CT. Mol Imaging Biol 22, 1111–1115 (2020). https://doi.org/10.1007/s11307-020-01482-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01482-z