Abstract
Background
There is conflicting results of few published human 18F-FDG PET/CT studies about BAT activation in breast cancer (BC). The aim of the study is to evaluate the association between the levels of BAT metabolic activity detected by 18F-FDG PET/CT and clinicopathological characteristics of a tumor in patients with primary BC.
Results
BAT was activated in 16 out of 157 (10.2%) consecutive female patients with BC who underwent 18F-FDG PET/CT for initial evaluation. The majority of patients (15/16) had bilateral uptake in the supraclavicular regions. The mean values of the highest SUVmax and total metabolic activity (TMA) of activated BAT were 13.3 ± 9.9 and 79.6 ± 45, respectively. Median outdoor temperature was significantly lower in the activated BAT group (P value=0.035). Patients with BAT activation tended to have a lower median primary tumor size and primary SUVmax, but not statistically significant than those without BAT activation. BAT activation was significantly more frequent among younger age groups (14/16) and patients with lower body mass index (BMI) (10/16), but it was insignificantly more frequent among estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), human epidermal growth factor receptor2 negative (HER2-), invasive ductal carcinoma (IDC), grade II, luminal B subtype, high Ki-67 expression level, patients with positive nodal metastasis, and in patients without distant metastasis. TMA was significantly higher among HER2+ patients (P value=0.019), but insignificantly higher among the younger age groups, stages I and II, invasive lobular carcinoma (ILC), grade I, luminal B subtype, ER+, PR−, higher Ki-67 expression level, patients with positive nodal, and distant metastasis. BMI and patient’s age were the significant independent predictor factors for BAT activation on multivariate regression analysis.
Conclusion
BAT activation in young age females is sex hormone-dependent, positively associated with less aggressive molecular subtypes of BC, less frequent in patients with distant metastasis. BAT activation may be a prognostic factor that carries a better prognosis in BC.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Worldwide, breast cancer (BC) is the most commonly diagnosed cancer among women, (excluding non-melanoma skin cancer) [1], and is the second leading cause of cancer-related death in women after lung cancer [2].
So, understanding the biological mechanisms that could affect the progression of this disease could yield new targets for prevention and treatment of advanced stages of BC with subsequently improved survival [3].
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is a non-invasive imaging modality used for staging and surveillance of various types of malignancies including BC through imaging of glucose metabolism in cancer cells [4]. Yet, 18F-FDG uptake is not tumor-specific; and several studies have reported a particular pattern of the neck and supraclavicular 18F-FDG uptake without any associated radiologically or clinically detectable pathology [5,6,7]. Brown adipose tissue (BAT) has been demonstrated to account for this pattern of uptake [8].
BAT is a thermoregulatory organ that consumes stored energy to produce heat through the expression of uncoupling protein 1(UCP1), a process called non-shivering thermogenesis and plays a role in glucose and lipid metabolism [9]. It is particularly abundant in newborns to maintain normal body temperatures. Although the prevalence of BAT declines with age, islets of brown adipocytes remain in the white adipose tissue of adult humans [10]. It is activated by cold exposure, with a higher prevalence in young, women, and lean subjects [11].
A few human 18F-FDG-PET/CT published studies suggested that BAT may play an important role in the development and progression of cancer [3, 4, 9, 10, 12]. However, their results are somewhat conflicting, while some of them suggested that BAT activity is greater in patients with active cancer [3, 9, 10], and higher BAT volume was associated with an increased risk of tumor recurrence/tumor-associated mortality independent of other risk factors, such as gender, age, body mass index (BMI), or tumor type [13]. The other reported a better prognosis of cancer patients with activated BAT [4, 12].
Two recent studies claimed that BAT activation may be a good prognostic factor in BC, one found a positive correlation between BAT 18F-FDG uptake with both human epidermal growth factor receptor 2 (HER2) and progesterone receptor (PR) expression and a negative one with estrogen receptor (ER) expression [4]; and the other found a higher prevalence of activated BAT in luminal B molecular subtype [12].
The aim of the present study was to evaluate the association between the level of BAT metabolic activity detected by PET/CT and clinicopathological characteristics of a tumor in patients with primary BC.
Methods
Study population
This retrospective study was approved by the local Institutional Committee of Medical Ethics with a waiver of consent.
Among all patients with newly diagnosed BC studied with 18F-FDG PET/CT at our institution between January 2017 and May 2020, female patients older than 18 years with available histological data were retrospectively selected. The study comprised 157 consecutive patients with histologically proven breast carcinoma (mean age 50.3 ±12.6 years, range 19–88).
Patient preparation and 18F-FDG PET/CT protocol
All patients underwent 18F-FDG PET/CT imaging after fasting for at least 4–6 h and had blood glucose <180 mg/dl immediately prior to IV administration of approximately 5.18 MBq/kg (0.14 mCi/kg) of 18F-FDG, with a maximum dose of 444 MBq (12 mCi) of 18F-FDG. During the subsequent 60 min following injection (uptake phase), patients were advised to remain seated or recumbent calmly in a comfortable quiet room, covered with a blanket to avoid uptake of the radiotracer at physiological sites as brown fat, which can result in image artifacts. Oral hydration with approximately 1000 ml of water and intravenous injection of furosemide 20 mg was performed after 18F-FDG administration. Patients were asked to empty their bladder immediately before the scan.
During image acquisition, patients were instructed to avoid motion and were allowed to breathe normally without specific instructions.
18F-FDG PET/CT image acquisition and reconstruction
Imaging was performed 60 min after 18F-FDG injection, using a high-spatial-resolution, full-ring PET scanner (Biograph mCT Flow, Siemens Healthcare, Erlangen, Germany), combining Lutetium oxyorthosilicate (LSO)-based PET crystals, and 20-slice CT components. An imaging field of view from the base of the skull to mid-thighs with the arms above the head whenever possible was used; otherwise, the arms were positioned beside the body.
The CT scan was performed before the emission acquisition as a single sweep. Here, slice thickness was 3 mm with a pitch of 0.9 and a tube voltage of 120 kV. The tube current automatically modulated according to the patient’s body mass index; a 50–100 mAs was used to achieve good image quality. CT data were used for image fusion and the generation of the CT transmission map. No intravenous contrast was used. PET emission data were acquired in a three-dimensional mode, using continuous table motion (CTM) acquisition mode with an average table speed of 0.9 mm/s.
The imaging data were reconstructed using a point spread function and a time-of-flight algorithm (TrueX + time-of-flight, UltraHD-PET), with 2 iterations and 21 subsets. Subsequently, a Gaussian filter with 5-mm full-width half-maximum was applied to the reconstructed images. For attenuation and scatter correction as well as anatomical mapping, a low-dose CT without contrast agent was used. Transaxial, sagittal, and coronal images as well as fused images were analyzed on manufacturer’s workstation (Syngo.via, Siemens Healthcare).
18F-FDG PET/CT image analysis, activated BAT identification, and semi-quantitative evaluation
Retrospective visual and semi-quantitative 18F-FDG PET/CT image analyses were performed in consensus by two experienced nuclear medicine physicians. 18F-FDG uptake in BAT was considered positive when the neck and/or supraclavicular and/or mediastinal and/or paravertebral foci with an uncommonly high 18F-FDG uptake were observed, which corresponding to adipose tissue attenuation on CT images (CT density − 250/−50 Hounsfield units). For each area showing active 18F-FDG BAT uptake, the body weight-normalized maximum standardized uptake value (SUVmax) was calculated by creating a 3-dimensional volume of interest (VOI) assigned to the area of the active focus manually on fused PET/CT images, using PET Volume Computerized-Assisted Reporting on Syngo.via workstation (Siemens Healthcare). In addition, the volume of activated BAT for each region was computed and the total metabolic activity (TMA) was recorded.
The distribution of activated BAT, age, BMI, fasting blood sugar, and gender of patients with activated BAT were recorded.
Axillary lymph nodes showing 18F-FDG uptake greater than the mediastinal blood pool were considered positive for metastasis on PET/CT and confirmed by biopsy or dissection. Distant metastasis was considered if there is any focal abnormal increased 18F-FDG uptake in any part of the scanned skeleton not explained by clinical relevant alternative and or with characteristic CT findings and confirmed by biopsy, another modality (CT or MRI) or follow-up study.
The patient population was divided into two groups according to their age at the time of diagnosis: <50 years and ≥50 years and into two groups according to BMI: BMI <25 and ≥25. The Ki-67 index was categorized as <14% and ≥14%, and subsequently, primary tumors were classified as luminal A, luminal B, HER2 overexpression (HER2+), and triple-negative (TN) subtypes.
Statistical analysis
Data were analyzed using SPSS 20.0 software (Statistical Package for the Social Sciences, IBM Inc., Armonk, NY). Continuous parametric variables were expressed as the mean ± standard deviation (SD). Non-parametric variables were displayed as the median and interquartile range (IQR). Categorical data are reported as percentages. Comparisons between continuous variables were performed with an independent sample t test or one-way ANOVA; Mann-Whitney U test was used to compare the medians. Statistical analysis of the categorical variables was conducted using a chi-square test. Person correlation analysis was used to assess the correlation between highest BAT SUVmax as well as TMA; and age, hormone receptor status, level of Ki-67 expression, BMI, outdoor temperature, primary tumor size, and primary SUV max; where the degree of correlation was considered as low (0–0.25), moderate (0.5–0.75), or high (0.75–1). Univariate and multivariate regression analysis was performed to identify the most powerful PET metabolic parameters to predict BAT activation. A P value < 0.05 was considered as statistically significant in all instances.
Results
A total of 157 consecutive female patients with invasive BC were enrolled in this retrospective study, mean age was 50.3 ± 12.6 years (range: 19–88). The clincopathological features of the study population are illustrated in Table 1.
BAT was activated in 16 of the 157 patients (10.2 %), with the majority of patients (15/16) had bilateral 18F-FDG uptake in the supraclavicular regions (Figs. 1, 2, 3, and 4). Three patients had uptake in four regions (two patients had BAT 18F-FDG uptake in cervical, supraclavicular, mediastinal, and paravertebral regions; and one patient had BAT 18F-FDG uptake in cervical, supraclavicular, paravertebral, and perinephric regions). Seven patients had uptake in three regions (four patients had BAT 18F-FDG uptake in cervical, supraclavicular, and paravertebral regions; and three patients had BAT 18F-FDG uptake in cervical, supraclavicular, and mediastinal regions). Three patients had uptake in two regions (two patients had BAT uptake in supraclavicular and mediastinal regions, and one patient had BAT 18F-FDG uptake in supraclavicular and paravertebral regions), and three patients had uptake in one region (two patients had BAT 18F-FDG uptake in supraclavicular region and one patient had BAT 18F-FDG uptake in paravertebral region).
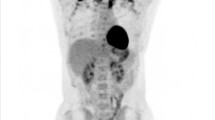
Brown fat uptake: 18F-FDG PET/CT scan of a 45-year-old female with luminal A invasive ductal carcinoma of the right breast: a maximum intensity projection, b transaxial PET show intense 18F-FDG uptake in the cervical, supraclavicular, paravertebral, and mediastinal regions. c The corresponding CT and d transaxial PET/CT fusion images reveal that 18F-FDG uptake is corresponding to fatty tissue, and there is no abnormal lymph node or muscle uptake, denoting activated BAT. The last row show intense FDG-avid primary lesion at the lower outer quadrant of the right breast with a metallic clip in situ, as well as foci of activated BAT at paravertebral, and mediastinal regions
Brown fat uptake: 18F-FDG PET/CT scan of a 46-year-old female with luminal B invasive lobular carcinoma of the left breast: a maximum intensity projection and b coronal PET show intense 18F-FDG uptake in the cervical, supraclavicular, and paravertebral regions. c The corresponding CT and d coronal PET/CT fusion images reveal that 18F-FDG uptake is corresponding to fatty tissue and there is no abnormal lymph node or muscle uptake, denoting activated BAT
Brown fat uptake: 18F-FDG PET/CT scan of a 41-year-old female with HER2 overexpression left breast cancer: a maximum intensity projection and b coronal PET show intense 18F-FDG uptake in the cervical, supraclavicular, paravertebral, and perinephric regions. c The corresponding CT and d coronal PET/CT fusion images reveal that 18F-FDG uptake is corresponding to the fatty tissue and there is no abnormal lymph node or muscle uptake, denoting activated BAT
Brown fat uptake: 18F-FDG PET/CT scan of a 28-year-old female with basal-like right breast cancer: a maximum intensity projection and b coronal PET show intense 18F-FDG uptake in the cervical, supraclavicular, and paravertebral regions. c The corresponding CT and d coronal PET/CT fusion images reveal that 18F-FDG uptake is corresponding to fatty tissue and there is no abnormal lymph node or muscle uptake, denoting activated BAT
The mean value of highest BAT SUVmax was 13.3 ± 9.9, and the mean value of BAT TMA was 79.6 ± 45 (Table 2).
Median outdoor temperature was significantly lower in the activated BAT group; 26 versus 34 (P value=0.035). Patients with BAT activation tended to have a lower median primary tumor size and primary SUVmax, but not statistically significant than those without BAT activation.
BAT activation was significantly more frequent among younger age groups (14/16) and patients with lower BMI (10/16), but it was insignificantly more frequent among ER+, PR+, HER2−, invasive ductal carcinoma (IDC), grade II, luminal B subtype, high Ki-67 expression level, patients with positive nodal metastasis, and in patients without distant metastasis (M0) as illustrated in Table 1.
Mean SUVmax was insignificantly higher in younger age group, stages III and IV, invasive lobular carcinoma (ILC), grade I, luminal A subtype, ER+, PR+, Her2−, patients with positive nodal metastasis, and patients without distant metastasis (M0).
TMA was significantly higher among HER2 positive patients (P value=0.019), but insignificantly higher among the younger age groups, stages I and II, ILC, grade I, luminal B subtype, ER+, PR−, higher Ki-67 expression level, patients with positive nodal metastasis, and patients with distant metastasis (M1) (Table 3).
The highest SUVmax was insignificantly negatively correlated with the age of patients in years, Ki-67 level, BMI, and tumor size (r=− 0.228, − 0.071, and − 0.175 with P value=0.396, 0.480, 0.517, and 0.738, respectively), while TMA was insignificantly positively correlated with Ki-67 and BMI (r=0.399 and 0.255 with P value = 0.287 and 0.36, respectively). Both highest SUVmax and TMA were insignificantly positively correlated with SUVmax of the primary tumor (Table 4).
Uni- and multivariate analyses
In univariate analysis, the patient’s age, weight in kilograms, BMI, and outdoor temperature were significantly associated with BAT activation. Before the multivariate regression, we performed a collinearity diagnostics of the aforementioned independent variables. We found that there was strong collinearity between the patient’s weight and BMI. Eventually, we included patient age, BMI, and outdoor temperature in the multivariate regression analysis. The result demonstrated that BMI (odds ratio [OR] = 3.779, P = 0.025), and patient’s age (OR=0.195, P=0.043) were the significant independent predictor factors for BAT activation in BC (Table 5).
Discussion
Metabolically active BAT has been recognized in adult humans after the advent of PET/CT [11, 14, 15]. Different studies have confirmed that adipose tissue does not only store energy but also secretes adipokines, inflammatory cytokines, growth factors, and free fatty acids, which can contribute to tumor growth and cancer progression [16,17,18]. Moreover, BAT has a similar biological property of hypermetabolism to cancer cells, this allowed in vivo quantification of BAT metabolism, which provided a major advancement in the field of BAT biology [19]. On 18F-FDG PET/CT imaging, BAT is most often detected in the cervical, supraclavicular, mediastinal, and paravertebral regions, and less frequently in the sub-phrenic areas [12]. In accordance, we found among patients with positive BAT, 15/16 (93.75%) patients had active BAT in the cervical, supraclavicular, mediastinal, and thoracic paravertebral regions, and only one (6.25%) patient had active perinephric BAT (sub-phrenic) as well.
Consistent with previous studies [10, 12], the current study showed that BAT activation is significantly associated with younger age, lower BMI, and lower outdoor temperature (P=0.001, 0.001, and 0.035), respectively. On the other hand, other studies found no significant difference between the BMI of patients with metabolically active BAT and that of patients without BAT [5, 8, 20]. Also, in a study done by Fujii et al. [4], discussing the association between BAT and BC, they reported no statistically significant association between the intensity of supraclavicular BAT activity and age or BMI of the patients (P=0.910 and 0.146), respectively.
The incidence of hypermetabolic BAT identified by 18F-FDG PET/CT has been reported in 1.7–9.3% of patients [21]. However, a relatively higher prevalence of activated BAT was found in patients with lymphoma (17%) and BC (15.2–80%) [3, 6, 10, 12, 22]. Our study demonstrated a lower prevalence (10.2%) of active BAT in the included BC patients, this difference could be explained by patients mismatching for age and weight (active versus non-active BAT groups). While in the other studies, analysis of BAT activity was based on age and body weight-matched groups of patients [3, 4, 12].
Given the retrospective nature of our study, we could not obtain the information about the menopausal status for each patient, to overcome this challenge, we stratified the data by age (<50 and ≥ 50 years old) to roughly classify the patients into premenopausal and postmenopausal groups, respectively.
Considering the positive BAT group, we found that active BAT is significantly prevalent in patients <50 years (14/16, 87.5%) versus (2/16, 12.5%) in patients ≥ 50 years, (P=0.001). In addition, the multivariate analysis revealed that BMI and age of the patients were the only factors significantly associated with BAT activity (P= 0.025 and 0.043, respectively). Furthermore, we found a more frequent expression of PR and ER in patients with active BAT, in consistent with other studies which documented higher prevalence of hypermetabolic BAT in non-menopausal patients and suggested that BAT metabolism is sex hormone-dependent and female sex hormones promote 18F-FDG uptake in BAT [6, 23, 24].
Our patients with active BAT showed insignificantly higher prevalence of lymph node metastasis and a lower prevalence of distant metastasis compared to patients without BAT activation. Also, TMA and SUVmax of BAT were insignificantly higher in patients with nodal metastasis. However, we demonstrated a significant positive association between SUVmax of BAT and absence of distant metastasis (P=0.021), this comes in contrast to Pace et al .[12], who documented that patients with active BAT had a significantly less frequent nodal and distant metastasis than in patients without active BAT (P < 0.05). Yet, in agreement with our findings, they stated the absence of significant difference in TMA and SUVmax of BAT between patients with and without nodal metastasis.
Moreover, we revealed that active BAT is more frequent in luminal B subtype, HER2−, and grade II, with higher activity of BAT in grade I. Both SUVmax and TMA tended to be higher in less aggressive molecular subtypes (luminal A and B), respectively. Although TMA of BAT was significantly higher among HER2+ patients (P=0.019), in the setting of HER2-targeted therapy, HER2+ BC is no longer associated with poor prognosis [25].
In the present study, we found a negative association between both metabolic activity (SUVmax), size of the primary tumor, and the prevalence of BAT activity, both of them tend to be lower in activated BAT group. Also, there was an insignificant correlation between the intensity of metabolic status of the primary tumor and that of BAT. In contrast, other studies found a positive association [4, 9, 10].
Our study had some limitations, the first one being a retrospective analysis, a small number of BAT-positive patients; other factors known to be associated with BAT activation including psychological factors and catecholamine levels and medical history of patients were not evaluated. A large prospective multicenter study is needed to verify the relationship between BAT activation and the long-term outcome of BC patients.
Conclusions
In summary, our findings support that the presence of BAT activation in young age females is sex hormone-dependent, positively associated with less aggressive molecular subtypes of BC, less frequent in patients with distant metastasis. So, we suggest that BAT activation may be a prognostic factor that carries a better prognosis in BC.
Availability of data and materials
Not applicable
Abbreviations
- 18F-FDG PET/CT:
-
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- BAT:
-
Brown adipose tissue
- BC:
-
Breast cancer
- BMI:
-
Body mass index
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- IDC:
-
Invasive lobular carcinoma
- ILC:
-
Invasive ductal carcinoma
- IQR:
-
Interquartile range
- PR:
-
Progesterone receptor
- SD:
-
Standard deviation
- SUVmax:
-
Maximum standardized uptake value
- TMA:
-
Total metabolic activity
- VOI:
-
Volume of interest
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 68:394–424
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A et al (2019) Breast cancer statistics, 2019. Cancer J Clin 69:438–451
Cao Q, Hersl J, La H, Smith M, Jenkins J, Goloubeva O et al (2014) A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 14:126
Fujii T, Yajima R, Tatsuki H, Oosone K, Kuwano H (2017) Implication of atypical supraclavicular F18-fluorodeoxyglucose uptake in patients with breast cancer: Association between brown adipose tissue and breast cancer. Oncol Lett 14:7025–7030
Cohade C, Osman M, Pannu HK, Wahl RL (2003) Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nuclear Med 44:170–176
Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal J et al (2006) Brown fat in breast cancer patients: analysis of serial 18 F-FDG PET/CT scans. Eur J Nuclear Med Mol Imaging 33:785–791
Cohade C, Mourtzikos KA, Wahl RL (2003) “USA-Fat”: prevalence is related to ambient outdoor temperature—evaluation with 18F-FDG PET/CT. J Nuclear Med 44:1267–1270
Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW et al (2004) Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. Am J Roentgenol 183:1127–1132
Bos SA, Gill CM, Martinez-Salazar EL, Torriani M, Bredella MA (2019) Preliminary investigation of brown adipose tissue assessed by PET/CT and cancer activity. Skeletal Radiol 48:413–419
Huang Y-C, Chen T-B, Hsu C-C, Li S-H, Wang P-W, Lee B-F et al (2011) The relationship between brown adipose tissue activity and neoplastic status: an 18 F-FDG PET/CT study in the tropics. Lipids Health Dis 10:238
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al (2009) Identification and importance of brown adipose tissue in adult humans. New Engl J Med 360:1509–1517
Pace L, Nicolai E, Basso L, Garbino N, Soricelli A, Salvatore M (2020) Brown adipose tissue in breast cancer evaluated by [18 F] FDG-PET/CT. Mol Imaging Biol 22:1111–1115
Chu K, Bos SA, Gill CM, Torriani M, Bredella MA (2020) Brown adipose tissue and cancer progression. Skeletal Radiol 49:635–639
Pelttari H, SCHALIN-JÄNTTI C, Arola J, Löyttyniemi E, Knuutila S, Välimäki MJ (2012) BRAF V600E mutation does not predict recurrence after long-term follow-up in TNM stage I or II papillary thyroid carcinoma patients. Apmis 120:380–386
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al (2009) Functional brown adipose tissue in healthy adults. New Engl J Med 360:1518–1525
Nieman KM, Romero IL, Van Houten B, Lengyel E (2013) Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of. Lipids 1831:1533–1541
Dittmer J, Leyh B (2014) Paracrine effects of stem cells in wound healing and cancer progression. Int J Oncol 44:1789–1798
Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM (2017) Signals from the adipose microenvironment and the obesity–cancer link—a systematic review. Cancer Prev Res 10:494–506
Bakhshayeshkaram M, Aghahosseini F, Dehghani Z, Doroudinia A, Hassanzad M, Ansari M et al (2018) Brown adipose tissue at F-18 FDG PET/CT: correlation of metabolic parameter with demographics and cancer-related characteristics in cancer patients. Iran J Radiol 15:e56074
Yeung HW, Grewal RK, Gonen M, Schöder H, Larson SM (2003) Patterns of 18F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nuclear Med 44:1789–1796
Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F et al (2014) Molecular imaging of brown adipose tissue in health and disease. Eur J Nuclear Med Mol Imaging 41:776–791
Döbert N, Menzel C, Hamscho N, Wördehoff N (2004) Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Q J Nuclear Med Mol Imaging 48:33
Rodriguez A, Monjo M, Roca P, Palou A (2002) Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol Life Sci 59:1714–1723
Bartness TJ, Wade GN (1984) Effects of interscapular brown adipose tissue denervation on body weight and energy metabolism in ovariectomized and estradiol-treated rats. Behav Neurosci 98:674
Figueroa-Magalhães MC, Jelovac D, Connolly RM, Wolff AC (2014) Treatment of HER2-positive breast cancer. Breast 23:128–136
Acknowledgements
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafa, N.M., Mohamadien, N.R.A. & Sayed, M.H.M. Brown adipose tissue (BAT) activation at 18F-FDG PET/CT: correlation with clinicopathological characteristics in breast cancer. Egypt J Radiol Nucl Med 52, 62 (2021). https://doi.org/10.1186/s43055-021-00438-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00438-9