Abstract
Purpose
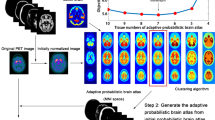
The Statistical Parametric Mapping (SPM) software is frequently used for the quantitative analysis of patients’ brain images obtained from 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography ([18F]FDG PET). However, its adaptation to small animals is difficult, particularly for the initial step of spatial normalization which requires a specific brain anatomical template. This study was aimed at determining whether SPM analysis can be applied to rat, and more specifically to the lithium-pilocarpine model of epilepsy, by using an adaptive template. This template developed for PET clinical imaging is constructed from a block matching algorithm.
Procedures
SPM analysis of brain [18F]FDG PET images from Sprague-Dawley rats was used with the block matching (BM) adaptive template for the detection of brain abnormalities (1) artificially inserted within the initially normal brain images of 10 rats (50 % decrease in signal intensity within 40 spheres of 0.5 to 1.0 mm in diameter) and (2) occurring at 4 h (n = 16), 48 h (n = 15), and 8 days (n = 13) after lithium-pilocarpine treatment.
Results
Concordant positive clusters were documented for all inserted abnormalities, whereas no aberrant clusters were documented in remote brain areas. Positive clusters were also detected on sites known to be involved in the epileptogenesis process of the lithium-pilocarpine model (piriform and entorhinal cortex, hippocampus), with the expected time-specific changes involving an early hypermetabolism followed by a severe hypometabolism and a subsequent partial recovery.
Conclusion
A quantitative SPM analysis of brain [18F]FDG PET images may be applied to the monitoring of rat brain function when using an adaptive BM template.


Similar content being viewed by others
References
Goffin K, Dedeurwaerdere S, Van Laere K et al (2008) Neuronuclear assessment of patients with epilepsy. Semin Nucl Med 38:227–239
Willmann O, Wennberg R, May T, Woermann FG et al (2007) The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy: a meta-analysis. Seizure 16:509–520
Verger A, Yagdigul Y, Van Der Gucht A et al (2016) Temporal epilepsy lesions may be detected by the voxel-based quantitative analysis of brain FDG-PET images using an original block-matching normalization software. Ann Nucl Med 30:272–278
Verger A, Hossu G, Kearney-Schwartz A et al (2016) Grey-matter metabolism in relation with white-matter lesions in older hypertensive patients with subjective memory complaints: a pilot voxel-based analysis study. Cerebrovasc Dis 42:106–109
Van Der Gucht A, Verger A, Guedj E et al (2015) Age-related changes in FDG brain uptake are more accurately assessed when applying an adaptive template to the SPM method of voxel-based quantitative analysis. Ann Nucl Med 29:921–928
Casteels C, Vermaelen P, Nuyts J et al (2006) Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. J Nucl Med 47:1858–1866
Nie B, Liu H, Chen K et al (2014) A statistical parametric mapping toolbox used for voxel-wise analysis of FDG-PET images of rat brain. PLoS One 9:e108295
Buiter HJ, van Velden FH, Leysen JE et al (2012) Reproducible analysis of rat brain PET studies using an additional [18F]NaF scan and an MR-based ROI template. Int J Mol Imaging 2012:580717
Lee EM, Park GY, Im KC et al (2015) Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia 53:860–869
Goffin K, Van Paesschen W, Dupont P et al (2009) Longitudinal microPET imaging of brain glucose metabolism in rat lithium-pilocarpine model of epilepsy. Exp Neurol 217:205–209
Jupp B, Williams J, Binns D et al (2012) Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia 53:1233–1244
Guo Y, Gao F, Wang S et al (2009) In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience 162:972–979
Person C, Louis-Dorr V, Poussier S et al (2012) Voxel-based quantitative analysis of brain images from 18F-FDG PET with a block-matching algorithm for spatial normalization. Clin Nucl Med 37:268–273
Dubé C, Boyet S, Marescaux C et al (2001) Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol 167:227–241
Curia G, Longo D, Biagini G et al (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172:143–157
Good CD, Johnsrude IS, Ashburner J et al (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36
Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113
Bieth M, Lombaert H, Reader AJ et al (2013) Atlas construction for dynamic (4D) PET using diffeomorphic transformations. Med Image Comput Comput Assist Interv 16:35–42
Martino ME, de Villoria JG, Lacalle-Aurioles M et al (2013) Comparison of different methods of spatial normalization of FDG-PET brain images in the voxel-wise analysis of MCI patients and controls. Ann Nucl Med 27:600–609
Della Rosa PA, Cerami C, Gallivanone F et al (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12:575–593
Da Silva Fernandes MJ, Dubé C, Boyet S et al (1999) Correlation between hypermetabolism and neuronal damage during status epilepticus induced by lithium and pilocarpine in immature and adult rats. J Cereb Blood Flow Metab 19:195–209
André V, Dubé C, François J et al (2007) Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia 48:41–47
Welch A, Mingarelli M, Riedel G, Platt B (2013) Mapping changes in mouse brain metabolism with PET/CT. J Nucl Med 54:1946–1953
Acknowledgements
The authors wish to thank M. Bernard Chalon for his help in software development, M. Pierre Pothier (Les Services PM-SYS, Sherbrooke, Canada) for critical review of the manuscript, and the team Inria Sophia Antipolis – Méditerranée “Nef” for the providing of the BM software and of the computation cluster.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Lorraine University through a dedicated Nancyclotep call for proposal and by the French National Health Ministry (“Yearly research funding for residents”).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was approved by the Lorraine Ethics Committee on Animal Experimentation (CELMEA LORRAIN N°66, Study Number 2015090117136919 (APAFIS # 1129)).
Additional information
Sylvain Poussier and Fatiha Maskali contributed equally to this article
Rights and permissions
About this article
Cite this article
Poussier, S., Maskali, F., Vexiau, G. et al. Quantitative SPM Analysis Involving an Adaptive Template May Be Easily Applied to [18F]FDG PET Images of the Rat Brain. Mol Imaging Biol 19, 731–735 (2017). https://doi.org/10.1007/s11307-016-1043-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-1043-9