Abstract
Purpose
Intraoperative optical imaging to guide surgeons during oncologic resections offers a unique and promising solution to the ambiguity of cancer margins to tactile and visual assessment that results in devastatingly high rates of positive margins. Sequestering of labeled antibodies by normal tissues with high expression of the antibody target, or “antigen sinks”, diminishes the efficacy of these probes to provide contrast between the tumor and background tissues by decreasing the amount of circulating probe available for uptake by the tumor and by increasing the fluorescence of non-tumor tissues. We hypothesized that administering a dose of unlabeled antibody prior to infusion of the near-infrared (NIR) fluorescently labeled antibody would improve tumor-specific uptake and contrast of the fluorescently labeled probe by occupying extra-tumoral binding sites, thereby increasing the amount of labeled probe available for uptake by the tumor.
Procedures
In this study, we explore this concept by testing two different “pre-load” doses of unlabeled cetuximab (the standard 10-mg test dose, and a larger, experimental 100-mg test dose) in six patients receiving cetuximab conjugated to the fluorescent dye IRDye800CW (cetuximab-IRDye800CW) in a clinical trial, and compared the amount of fluorescent antibody in tumor and background tissues, as well as the tumor-specific contrast of each.
Results
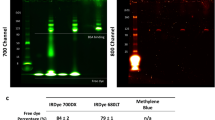
The patients receiving the larger preload (100 mg) of unlabeled cetuximab demonstrated significantly higher concentrations (9.5 vs. 0.1 μg) and a longer half-life (30.3 vs. 20.6 days) of the labeled cetuximab in plasma, as well as significantly greater tumor fluorescence (32.3 vs. 9.3 relative fluorescence units) and tumor to background ratios (TBRs) (5.5 vs. 1.7).
Conclusions
Administering a preload of unlabeled antibody prior to infusion of the fluorescently labeled drug may be a simple and effective way to improve the performance of antibody-based probes to guide surgical resection of solid malignancies.


Similar content being viewed by others
References
Woolgar JA, Triantafyllou A (2005) A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol 41:1034–1043
McMahon J, O’Brien CJ, Pathak I et al (2003) Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg 41:224–231
Hinni ML, Ferlito A, Brandwein-Gensler MS et al (2013) Surgical margins in head and neck cancer: a contemporary review. Head Neck 35:1362–1370
Stummer W, Novotny A, Stepp H et al (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Rosenthal EL, Warram JM, de Boer E et al (2016) Successful translation of fluorescence navigation during oncologic surgery: a consensus report. J Nucl Med 57:144–150
Koch M, Ntziachristos V (2016) Advancing surgical vision with fluorescence imaging. Annu Rev Med 67:153–164
Rosenthal EL, Warram JM, de Boer E et al (2015) Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res 21:3658–3666
Whitley MJ, Cardona DM, Lazarides AL et al (2016) A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med 8:320ra324
Day KE, Sweeny L, Kulbersh B et al (2013) Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Mol Imaging Biol 15:722–729
Korb ML, Hartman YE, Kovar J et al (2014) Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res 188:119–128
Day KE, Beck LN, Deep NL et al (2013) Fluorescently labeled therapeutic antibodies for detection of microscopic melanoma. Laryngoscope 123:2681–2689
Kulbersh BD, Duncan RD, Magnuson JS et al (2007) Sensitivity and specificity of fluorescent immunoguided neoplasm detection in head and neck cancer xenografts. Arch Otolaryngol Head Neck Surg 133:511–515
Rosenthal EL, Kulbersh BD, Duncan RD et al (2006) In vivo detection of head and neck cancer orthotopic xenografts by immunofluorescence. Laryngoscope 116:1636–1641
Tanaka E, Choi HS, Humblet V et al (2008) Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery 144:39–48
Tanaka E, Ohnishi S, Laurence RG et al (2007) Real-time intraoperative ureteral guidance using invisible near-infrared fluorescence. J Urol 178:2197–2202
van der Vorst JR, Schaafsma BE, Verbeek FP et al (2013) Near-infrared fluorescence sentinel lymph node mapping of the oral cavity in head and neck cancer patients. Oral Oncol 49:15–19
Hutteman M, Choi HS, Mieog JS et al (2011) Clinical translation of ex vivo sentinel lymph node mapping for colorectal cancer using invisible near-infrared fluorescence light. Ann Surg Oncol 18:1006–1014
Tjalma JJ, Garcia-Allende PB, Hartmans E et al (2016) Molecular fluorescence endoscopy targeting vascular endothelial growth factor A for improved colorectal polyp detection. J Nucl Med 57:480–485
Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB et al (2011) Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med 52:1778–1785
Wu J, Ma R, Cao H et al (2013) Intraoperative imaging of metastatic lymph nodes using a fluorophore-conjugated antibody in a HER2/neu-expressing orthotopic breast cancer mouse model. Anticancer Res 33:419–424
Metildi CA, Kaushal S, Pu M et al (2014) Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer. Ann Surg Oncol 21:1405–1411
McElroy M, Kaushal S, Luiken GA et al (2008) Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg 32:1057–1066
Metildi CA, Tang CM, Kaushal S et al (2013) In vivo fluorescence imaging of gastrointestinal stromal tumors using fluorophore-conjugated anti-KIT antibody. Ann Surg Oncol 20(Suppl 3):S693–S700
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH (2010) Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49:493–507
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Mould DR, Green B (2010) Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs 24:23–39
Dostalek M, Gardner I, Gurbaxani BM et al (2013) Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 52:83–124
Greish K (2007) Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target 15:457–464
Greish K (2010) Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol 624:25–37
Mould DR, Sweeney KR (2007) The pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel 10:84–96
Owens EA, Lee S, Choi J, Henary M, Choi HS (2015) NIR fluorescent small molecules for intraoperative imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:828–838
Vira S, Mekhedov E, Humphrey G, Blank PS (2010) Fluorescent-labeled antibodies: balancing functionality and degree of labeling. Anal Biochem 402:146–150
McCombs JR, Owen SC (2015) Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J 17:339–351
Lammerts van Bueren JJ, Bleeker WK, Bogh HO et al (2006) Effect of target dynamics on pharmacokinetics of a novel therapeutic antibody against the epidermal growth factor receptor: implications for the mechanisms of action. Cancer Res 66:7630–7638
Ternant D, Bejan-Angoulvant T, Passot C et al (2015) Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin Pharmacokinet 54:1107–1123
Harding J, Burtness B (2005) Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today (Barc) 41:107–127
Adams CB, Street DS, Crass M, Bossaer JB (2015) Low rate of cetuximab hypersensitivity reactions in Northeast Tennessee: An Appalachian effect? J Oncol Pharm Pract
Zinn KR, Korb M, Samuel S et al (2015) IND-directed safety and biodistribution study of intravenously injected cetuximab-IRDye800 in cynomolgus macaques. Mol Imaging Biol 17:49–57
Heath CH, Deep NL, Beck LN et al (2013) Use of panitumumab-IRDye800 to image cutaneous head and neck cancer in mice. Otolaryngol Head Neck Surg 148:982–990
Kletting P, Meyer C, Reske SN, Glatting G (2010) Potential of optimal preloading in anti-CD20 antibody radioimmunotherapy: an investigation based on pharmacokinetic modeling. Cancer Biother Radiopharm 25:279–287
Muylle K, Flamen P, Vugts DJ et al (2015) Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur J Nucl Med Mol Imaging 42:1304–1314
de Boer E, Warram JM, Tucker MD et al (2015) In vivo fluorescence immunohistochemistry: localization of fluorescently labeled cetuximab in squamous cell carcinomas. Sci Rep 5:10169
Garkavij M, Tennvall J, Strand SE et al (1994) Improving radioimmunotargeting of tumors: the impact of preloading unlabeled L6 monoclonal antibody on the biodistribution of 125I-L6 in rats. J Nucl Biol Med 38:594–600
Kletting P, Kull T, Bunjes D et al (2011) Optimal preloading in radioimmunotherapy with anti-cD45 antibody. Med Phys 38:2572–2578
Acknowledgments
The authors acknowledge Ms. Yolanda Hartman and Ms. Lisa Clemons for their contributions to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
Funding for this manuscript includes the Robert Armstrong Research Acceleration Fund, the UAB Comprehensive Cancer Center, NIH/NCI (R21CA179171, R21CA182953, T32CA091078), and institutional equipment loans from Novadaq and LI-COR Biosciences.
Rights and permissions
About this article
Cite this article
Moore, L.S., Rosenthal, E.L., de Boer, E. et al. Effects of an Unlabeled Loading Dose on Tumor-Specific Uptake of a Fluorescently Labeled Antibody for Optical Surgical Navigation. Mol Imaging Biol 19, 610–616 (2017). https://doi.org/10.1007/s11307-016-1022-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-1022-1