Abstract
Purpose
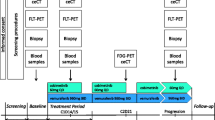
This study aims to develop a molecular imaging strategy for response assessment of arginine deiminase (ADI) treatment in melanoma xenografts using 3′-[18F]fluoro-3′-deoxythymidine ([18F]-FLT) positron emission tomography (PET).
Procedures
F-FLT response to ADI therapy was studied in preclinical models of melanoma in vitro and in vivo. The molecular mechanism of response to ADI therapy was investigated, with a particular emphasis on biological pathways known to regulate 18F-FLT metabolism.
Results
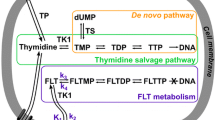
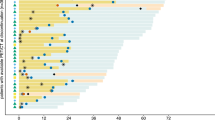
Proliferation of SK-MEL-28 melanoma tumors was potently inhibited by ADI treatment. However, no metabolic response was observed in FLT PET, presumably based on the known ADI-induced degradation of PTEN, followed by instability of the tumor suppressor p53 and a relative overexpression of thymidine kinase 1, the enzyme mainly responsible for intracellular FLT processing.
Conclusion
The specific pharmacological properties of ADI preclude using 18F-FLT to evaluate clinical response in melanoma and argue for further studies to explore the use of other clinically applicable PET tracers in ADI treatment.







Similar content being viewed by others
References
Izzo F, Marra P, Beneduce G et al (2004) Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 22:1815–1822
Ascierto PA, Scala S, Castello G et al (2005) Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol 23:7660–7668
Glazer ES, Piccirillo M, Albino V et al (2010) Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol 28:2220–6
Feun LG, Marini A, Walker G et al (2012) Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer 106:1481–5
Dillon BJ, Prieto VG, Curley SA et al (2004) Incidence and distribution of argininosuccinate synthetase deficiency in human cancers. Cancer 100:826–33
Kim RH, Coates JM, Bowles TL et al (2009) Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res 69:700–8
Kelly MP, Junbluth AA, Wu BW et al (2012) Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br J Cancer 106:324–32
Kobayashi E, Masuda M, Nakayama R et al (2010) Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther 9:535–44
Larson SM, Schwartz LH (2006) 18F-FDG as a candidate for “qualified biomarker”: functional assessment of treatment response in oncology. J Nucl Med 47:901–3
Stelter L, Evans MJ, Jungbluth AA et al (2012) Novel mechanistic insights into arginine deiminase pharmacology suggest 18F-FDG is not suitable to evaluate clinical response in melanoma. J Nucl Med 53:281–6
Ott PA, Carvajal RD, Pandit-Taskar N et al (2012) Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs 31(2):425–34. doi:10.1007/s10637-012-9862-2
Rasey JS, Grierson JR, Wiens LW et al (2002) Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 43:1210–17
Been LB, Suurmeijer AJH, Cobben DCP et al (2004) [18-F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Biol 31:1659–1672
Liu W, Zhou Y, Reske SN, Shen C (2008) PTEN Mutation: many birds with one stone in tumorigenesis. anticancer Res 28:3613–3620
Benz MR, Czernin J, Allen-Auerbach MS et al (2012) 3′-Deoxy-3′-[18F] fluorothymidine positron emission tomography for response assessment in soft tissue sarcoma. Cancer 118:3135–44
Savaraj N, You M, Wu C et al (2010) Arginine deprivation, autophagy, apoptosis (AAA) for the treatment of melanoma. Curr Mol Med 10:405–412
Syed N, Langer J, Janczar K et al (2013) Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis 4:e458
Delage B, Luong P, Maharaj L et al (2012) Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis 3:e342
You M, Savaraj N, Wangpaichitr M et al (2010) The combination of ADI-PEG20 and TRAIL effectively increases cell death in melanoma cell lines. Biochem Biophys Res Com 394:760–76
Lucignani G, Larson SM (2010) Doctor, what does my future hold? The prognostic value of FDG-PET in solid tumours. Eur J Nucl Med Mol Imaging 37:1032–8
Larson SM, Robbins R (2002) Positron emission tomography in thyroid cancer management. Semin Roentgenol 37:169–74
Lu SJ, Gnanasegaran G, Buscombe J, Navalkissoor S (2013) Single photon emission computed tomography/computed tomography in the evaluation of neurendocrine tumours: a review of the literature. Nucl Med Commun 34:98–107
Stelter L, Evans MJ, Junbluth AA et al (2013) Imaging of tumor vascularization using fluorescence molecular tomography to monitor arginine deiminase treatment in melanoma. Mol Imaging 12:67–73
Szlosarek PW, Luong P, Phillips MM et al (2013) Metabolic response to pegylated arginine deiminase in mesothelioma with promoter methylation of argininosuccinate synthetase. J Clin Oncol 31:1–3
Hoshikawa H, Nishiyama Y, Kishino T et al (2011) Comparison of FLT-PET and FDG-PET for visualization of head and neck squamous cell cancers. Mol Img Biol 13:172.7
Fatema CN, Zhao S, Zhao Y et al (2013) Monitoring tumor proliferation response to radiotherapy using (18)F-fluorothymidine in human head and neck cancer xenograft in comparison with Ki-67. Ann Nucl Med 27(4):355–62. doi:10.1007/s12149-013-0693-9
Sherley JL, Kelly TJ (1988) Regulation of human thymidine kinase during the cell cycle. J Biol Chem 263:8350–8358
Wang H, He Q, Skog S, Eriksson S, Tribukait B (2001) Investigation on cell proliferation with a new antibody against thymidine kinase 1. Anal Cell Pathol 23:11–19
De Saint-Hubert M, Brepoels L, Devos E et al (2012) Molecular imaging of therapy response with (18)F-FLT and (18)F-FDG following cyclophosphamide and mTOR inhibition. Am J Nucl Med Mol Img 1:110–121
Feun L, Savaraj N (2006) Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs 15:815–822
Mayo LD, Dixon DB, Durden DL et al (2002) PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J Biol Chem 277:5484–9
Schwartz JL, Tamura Y, Jordan R et al (2004) Effect of p53 activation on cell growth, thymidine kinase-1 activity, and 3′-deoxy-3′fluorothymidine uptake. Nucl Med Biol 31:419–423
Freeman DJ, Li AG, Wei G et al (2003) PTEN tumor suppressor regulates p53 protein levels and activity through phostphatase-dependant and -independent mechanisms. Cancer Cell 3:117–30
Cui X, Witalison EE, Chumanevich AP et al (2013) The induction of microRNA-16 in colon cancer cells by protein arginine deiminase inhibition cause a p53-dependent cell cycle arrest. PLoS One 8:e53791
Paproski RJ, Wuest M, Jans HS et al (2010) Biodistribution and uptake of 3′deoxy-3′-fluorothymidine in ENT1-knockout mice and in an ENT1-knockdown tumor model. J Nucl Med 51:1447–55
Plotnik DA, McLaughlin LJ, Chan J et al (2011) The role of nucleoside/nucleotide transport and metabolism in the uptake and retention of 3′-fluoro-3′-deoxythymidine in human B-lymphoblast cells. Nucl Med Biol 38:979–86
Plotnik DA, McLaughlin LJ, Krohn KA, Schwartz JL (2012) The effects of 5-fluoruracil treatment on 3′-fluoro-3′deoxythymidine (FLT) transport and metabolism in proliferating and non-proliferating cultures of human tumor cells. Nucl Med Biol 39:970–976
Höglund J, Shirvan A, Antoni G et al (2011) 18F-ML-10, a PET tracer for apoptosis: first human study. J Nucl Med 52:720–5
Sobrio F, Médoc M, Martial L et al (2013) Automated radiosynthesis of [(18)F]ML-10, a PET radiotracer dedicated to apoptosis imaging, on a TRACERLab FX-FN module. Mol Imaging Biol 15:12–8
Acknowledgments
The authors would like to thank Dr. Marcus Kelly for his informative discussions and Megan Holz for her invaluable assistance. We also thank the staff of the Radiochemistry/Cyclotron Core at MSKCC. Technical services provided by the MSKCC Small-Animal Imaging Core Facility were supported in part by NIH grants R24 CA83084 and P30 CA08748. L.S. was supported by a grant (Ste 1837/1-1) of the Deutsche Forschungsgemeinschaft.
Conflict of Interest
J.S.B. is the Executive Vice President of Polaris Group that provided ADI-PEG 20 for this study. All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stelter, L., Fuchs, S., Jungbluth, A.A. et al. Evaluation of Arginine Deiminase Treatment in Melanoma Xenografts Using 18F-FLT PET. Mol Imaging Biol 15, 768–775 (2013). https://doi.org/10.1007/s11307-013-0655-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0655-6