Abstract
Purpose
The phosphatidyl inositol 3 kinase, AKT and mammalian target of rapamycin are frequently deregulated in human cancer and are among one of the most promising targets for cancer therapy. AZD5363 (AstraZeneca) is an AKT inhibitor in phase 1 clinical trials. Given its utility in assessing glucose metabolism, we investigated the role of 2-Deoxy-2-[18F]fluoro-d-glucose (18F-FDG) positron emission tomography (PET) as a biomarker to demonstrate target inhibition and its potential to predict and demonstrate the anti-tumour activity of AZD5363.
Methods
18F-FDG PETscans were performed in nude mice in a number of xenograft models (U87-MG glioblastoma, BT474C breast carcinoma and Calu-6 lung). Mice were fasted prior to imaging, and either static or dynamic 18F-FDG PET imaging was performed.
Results
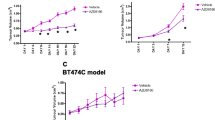
We have shown that 18F-FDG uptake in tumour xenografts was reduced by 39 % reduction compared to vehicle after a single dose of AZD5363, demonstrating activation of the AKT pathway after only 4 h of dosing. Multiple doses of AZD5363 showed an anti-tumour volume effect and a reduction in 18F-FDG uptake (28 % reduction compared to vehicle), highlighting the potential of 18F-FDG PET as an efficacy biomarker. Furthermore, the degree of inhibition of 18F-FDG uptake corresponded with the sensitivity of the tumour model to AZD5363. The use of dynamic 18F-FDG PET and a two-compartmental analysis identified the mechanism of this change to be due to a change in cellular uptake of 18F-FDG following administration of AZD5363.
Conclusions
We conclude that 18F-FDG PET is a promising pharmacodynamic biomarker of AKT pathway inhibition, with potential to predict and demonstrate anti-tumour activity. It is a biomarker that may stop ineffective drug schedules, helping to make early stop decisions and identify responding subsets of patients, resulting in improved clinical decision making both during drug development and patient management.






Similar content being viewed by others
References
Luo J, Manning BD, Cantley LC (2003) Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4:257–262
Marone R, Cmiljanovic V, Giese B, Wymann MP (2008) Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta 1784:159–185
Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27:5497–5510
Markman B, Dienstmann R, Tabernero J (2010) Targeting the PI3K/AKT/mTOR pathway beyond rapalogs. Oncotarget 1:530–543
Hirai H, Sootome H, Nakatsuru Y, Miyama K et al (2010) MK-2206, an allosteric AKT inhibitor, enhances tumour efficacy by standard chemotherapeutic agents or molecular targeted drugs in-vitro and in-vivo. Mol Cancer Ther 9:1956–1967
Davies BR, Greenwood H, Dudley P, Crafter C et al (2012) Preclinical Pharmacology of AZD5363: an inhibitor of AKT: pharmacodynamics, antitumour activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 11:873–887
Wahl RL, Zasadny K, Helvie M, Hutchins GD et al (1993) Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol 11:2101–2111
Kidd EA, Siegel BA, Dehdashti F, Grigsby PW (2007) The standardised uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer 15:1738–1744
Larson SM, Schwartz LH (2006) 18F-FDG PET as a candidate for “Qualified Biomarker”: functional assessment of treatment response in oncology. J Nucl Med 47:901–903
Stroobants S, Goeminne J, Seegers M, Dimitrijevic S et al (2003) 18FDG Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer 39:2012–2020
Sunaga N, Oricuhi N, Kaira K, Yanagitani N et al (2008) Usefulness of FDG-PET for early prediction of the response to Gefitinib in non small cell lung cancer. Lung Cancer 59:203–210
Bendell JC, Rodon J, Burris HA, de Jonge M et al (2012) Phase 1, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumours. J Clin Oncol 30:282–290
Young H, Baum R, Cremerius U, Herholz K et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organisation for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–1782
Roe K, Aleksandersen TB, Kristian A, Nilsen LB et al (2010) Pre-clinical dynamic 18F-FDG PET—tumour characterization and radiotherapy response assessment by kinetic compartment analysis. Acta Oncol 49:914–921
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 29:1261–1274
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R et al (2004) AKT stimulates aerobic glycolysis in cancer cells. Cancer Res 64:3892–3899
Ma WW, Jacene H, Song D, Vilardell F et al (2009) 18F Fluorodeoxyglucose positron emission tomography correlates with AKT pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol 27:2697–2704
Nogova L, Boellaard R, Kobe C, Hoetjes N et al (2009) Downregulation of 18F-FDG uptake in PET as an early pharmacodynamic effect in treatment of non-small cell lung cancer with the mTOR inhibitor everolimus. J Nucl Med 50:1815–1819
Bao Q, Newport D, Chen M, Stout D et al (2009) Performance evaluation of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J Nucl Med 50:401–408
Gambhir S (2004) Quantitative assay development for PET. In: Phelps ME (ed) PET: molecular imaging and its biological applications. Springer-Verlag, Berlin Germany, pp 125–216
Laforest R, Sharp TL, Engelbach JA, Fettia NM et al (2005) Measurement of input functions in rodents: challenges and solutions. Nucl Med Biol 32:679–685
Nguyen QD, Perumal M, Waldman TA, Abogaye EO (2011) Glucose metabolism measured by [18F] fluorodeoxyglucose positron emission tomography is independent of PTEN/AKT status in human colon carcinoma cells. Transl Oncol 4:241–248
Keen H, Ricketts SA, Bales J, Shannon A et al (2009) The mTOR kinase inhibitor AZD8055 modulates 18 F-FDG uptake in vivo in the human glioma xenograft model U87-MG. Mol Cancer Ther 8(supplement 1):A225
Wei LH, Su H, Hildebrandt IJ, Phelps ME et al (2008) Changes in tumour metabolism as readout for mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin Cancer Res 14:3416–3426
Nogova L, Gross SH, Dimitrijevic et al (2008). Pharmacodynamics of RAD001 measured by early FDG-PET in patients with recurrent NSCLC (abstract). J Clin Oncol 26(May 20 suppl): Abstract 14616
Contractor KN, Aboagye EO (2009) Monitoring predominantly cytostatic treatment response with 18F-FDG PET. J Nucl Med 50:97S–105S
Benz MR, Czernin J, Allen Auebrach MS, Itap UD et al (2009) FDG-PET/CT imaging predicts histopathological treatment responses after the initial cycle of neoadjuvant therapy in high grade soft-tissue sarcomas. Clin Cancer Res 15:2856–2863
De Geus Oei LF, Vriens D, Van Laarloven HW, Van der Graaf WT et al (2009) Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med 50:543–554
Cheebsumon P, Velasquez LM, Hoekstra CJ, Hayes W et al (2011) Measuring response to therapy using 18F-FDG PET. Semi-quantitative and full kinetic analysis. Eur J Nucl Med Mol Imaging 38:832–842
Minn H, Leskinen-Kallio S, Lindholm P, Bergmani J et al (1993) 18F Flourodeoxyglucose uptake in tumours. Kinetic vs steady state methods with reference to plasma insulin. J Comput Assist Tomgr 17:115–123
Minn H, Zasadny KR, Quint LE, Wahl RL (1995) Lung cancer: reproducibility of quantitative measurements for evaluating 2[F18]-fluorodeoxyglucose uptake of PET. Radiology 196:167–173
Plathow C, Weber WA (2008) Tumour cell metabolism imaging. J Nucl Med 49(Suppl2):43S–63S
Zhao S, Kuge Y, Mochizuki T, Takahashi T et al (2005) Biologic correlates of intra-tumoural heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase II in experimental tumour. J Nucl Med 46:675–682
Pugachev A, Ruan S, Carlin S, Larson SM et al (2005) Dependence of FDG uptake on tumour micro-environment. Int J Radiat Oncol Biol Phys 62:545–553
Tseng J, Dunnwald LK, Schubert EK, Link JM et al (2004) 18F-FDG kinetics in locally advanced breast cancer: correlation with tumour blood flow and changes in response to neoadjuvant chemotherapy. J Nucl Med 45:1829–1837
Phelps ME, Huang SC, Hoffman EJ, Selin MS et al (1999) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F18) 2-flourodeoxyglucose: validation of method. Ann Neurol 6:371–388
Acknowledgments
AZD5363 was discovered by AstraZeneca subsequent to a collaboration with Astex Therapeutics. The authors thank Neill Gingles, Leigh Williams, Gareth Parker and Heather Keen for their contribution to the in vivo imaging procedures.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maynard, J., Ricketts, SA., Gendrin, C. et al. 2-Deoxy-2-[18F]fluoro-d-glucose Positron Emission Tomography Demonstrates Target Inhibition with the Potential to Predict Anti-Tumour Activity Following Treatment with the AKT Inhibitor AZD5363. Mol Imaging Biol 15, 476–485 (2013). https://doi.org/10.1007/s11307-013-0613-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0613-3