Abstract
Purpose
The objective of this study was to track the fate of iron-labeled, multipotent stromal cells (MSC) after their direct transplantation into mice with spinal cord injuries using magnetic resonance imaging (MRI).
Procedures
Mice with spinal cord injuries received a direct transplant of (1) live MSC labeled with micron-sized iron oxide particles (MPIO); (2) dead, MPIO-labeled MSC; (3) unlabeled MSC; or (4) free MPIO and were imaged at 3 T for 6 weeks after transplantation.
Results
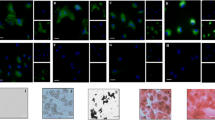
Live, iron-labeled MSC appeared as a well-defined region of signal loss in the mouse spinal cord at the site of transplant. However, the MR appearance of dead, iron-labeled MSC and free iron particles was similar and persisted for the 6 weeks of the study.
Conclusions
Iron-labeled stem cells can be detected and monitored in vivo after direct transplantation into the injured spinal cord of mice. However, the fate of the iron label is not clear. Our investigation indicates that caution should be taken when interpreting MR images after direct transplantation of iron-labeled cells.







Similar content being viewed by others
References
Dousset V, Tourdias T, Brochet B, Boiziau C, Petry KG (2008) How to trace stem cells for MRI evaluation? J Neurol Sci 265(1–2):122–126
Foster PJ, Dunn EA, Karl KE et al (2008) Cellular magnetic resonance imaging: in vivo imaging of melanoma cells in lymph nodes of mice. Neoplasia 10(3):207–216
Heyn C, Ronald JA, Ramadan SS et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56(5):1001–1010
Medarova Z, Tsai S, Evgenov N, Santamaria P, Moore A (2008) In vivo imaging of a diabetogenic CD8+ T cell response during type 1 diabetes progression. Magn Reson Med 59(4):712–720
Song M, Kim Y, Ryu S et al (2009) MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci Res 64(2):235–239
Sun JH, Teng GJ, Ju SH et al (2008) MR tracking of magnetically labeled mesenchymal stem cells in rat kidneys with acute renal failure. Cell Transplant 17(3):279–290
Walczak P, Zhang J, Gilad AA et al (2008) Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39(5):1569–1574
Wu YL, Ye Q, Foley LM et al (2006) In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A 103(6):1852–1857
Heyn C, Bowen CV, Rutt BK, Foster PJ (2005) Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med 53(2):312–320
Bulte JW, Ben-Hur T, Miller BR et al (2003) MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn Reson Med 50(1):201–205
Chang NK, Jeong YY, Park JS et al (2008) Tracking of neural stem cells in rats with intracerebral hemorrhage by the use of 3 T MRI. Korean J Radiol 9(3):196–204
Jendelova P, Herynek V, Urdzikova L et al (2004) Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res 76(2):232–243
Lee IH, Bulte JW, Schweinhardt P et al (2004) In vivo magnetic resonance tracking of olfactory ensheathing glia grafted into the rat spinal cord. Exp Neurol 187(2):509–516
Lepore AC, Walczak P, Rao MS, Fischer I, Bulte JW (2006) MR imaging of lineage-restricted neural precursors following transplantation into the adult spinal cord. Exp Neurol 201(1):49–59
Sykova, E. and Jendelova, P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ, 2007.
Sykova E, Jendelova P (2007) In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res 161:367–383
Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26(7–8):1167–1180
Koshizuka S, Okada S, Okawa A et al (2004) Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol 63(1):64–72
Louro J, Pearse DD (2008) Stem and progenitor cell therapies: recent progress for spinal cord injury repair. Neurol Res 30(1):5–16
Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K (2007) Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem 102(5):1459–1465
Okano H, Sawamoto K (2008) Neural stem cells: involvement in adult neurogenesis and CNS repair. Philos Trans R Soc Lond B Biol Sci 363(1500):2111–2122
Jackson L, Jones DR, Scotting P, Sottile V (2007) Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J Postgrad Med 53(2):121–127
Tropel P, Platet N, Platel JC et al (2006) Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells 24(12):2868–2876
Rapalino O, Lazarov-Spiegler O, Agranov E et al (1998) Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med 4(7):814–821
Schwartz M, Lazarov-Spiegler O, Rapalino O et al (1999) Potential repair of rat spinal cord injuries using stimulated homologous macrophages. Neurosurgery 44(5):1041–1045, discussion 1045-1046
Schwartz M (2001) Immunological approaches to the treatment of spinal cord injury. BioDrugs 15(9):585–593
Mikami Y, Okano H, Sakaguchi M et al (2004) Implantation of dendritic cells in injured adult spinal cord results in activation of endogenous neural stem/progenitor cells leading to de novo neurogenesis and functional recovery. J Neurosci Res 76(4):453–465
McDonald JW, Liu XZ, Qu Y et al (1999) Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5(12):1410–1412
Ogawa Y, Sawamoto K, Miyata T et al (2002) Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 69(6):925–933
Chopp M, Zhang XH, Li Y et al (2000) Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. NeuroReport 11(13):3001–3005
Hofstetter CP, Schwarz EJ, Hess D et al (2002) Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 99(4):2199–2204
Ohta M, Suzuki Y, Noda T et al (2004) Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol 187(2):266–278
Zurita M, Vaquero J (2004) Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. NeuroReport 15(7):1105–1108
Chernykh ER, Stupak VV, Muradov GM et al (2007) Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med 143(4):543–547
Deda H, Inci MC, Kurekci AE et al (2008) Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy 10(6):565–574
Moviglia, G.A., Varela, G., Brizuela, J.A.et al. 2009 Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord
Sykova E, Homola A, Mazanec R et al (2006) Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 15(8–9):675–687
Callera F, de Melo CM (2007) Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. Stem Cells Dev 16(3):461–466
Terrovitis J, Stuber M, Youssef A et al (2008) Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 117(12):1555–1562
Jacob JE, Gris P, Fehlings MG, Weaver LC, Brown A (2003) Autonomic dysreflexia after spinal cord transection or compression in 129 Sv, C57BL, and Wallerian degeneration slow mutant mice. Exp Neurol 183(1):136–146
Joshi M, Fehlings MG (2002) Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma 19(2):175–190
Koda M, Okada S, Nakayama T et al (2005) Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. NeuroReport 16(16):1763–1767
Kobayashi H, Kawamoto S, Star RA et al (2003) Micro-magnetic resonance lymphangiography in mice using a novel dendrimer-based magnetic resonance imaging contrast agent. Cancer Res 63(2):271–276
Rosset A, Spadola L, Ratib O (2004) OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 17(3):205–216
Blight AR (2002) Miracles and molecules—progress in spinal cord repair. Nat Neurosci 5(Suppl):1051–1054
Zhou B, Shan H, Li D (1996) MR tracking of magnetically labeled mesenchymal stem cells in rats with liver fibrosis. Magn Reson Imaging 28(3):394–399
Politi LS, Bacigaluppi M, Brambilla E et al (2007) Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells 25(10):2583–2592
Arbab AS, Janic B, Knight RA et al (2008) Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. FASEB J 22(9):3234–3246
Magnitsky S, Walton RM, Wolfe JH, Poptani H (2008) Magnetic resonance imaging detects differences in migration between primary and immortalized neural stem cells. Acad Radiol 15(10):1269–1281
McAteer MA, Sibson NR, von Zur Muhlen C et al (2007) In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat Med 13(10):1253–1258
Bernas LM, Foster PJ, Rutt BK (2007) Magnetic resonance imaging of in vitro glioma cell invasion. J Neurosurg 106(2):306–313
Cromer Berman, S.M., Gilad, A.A., Bulte, J.W. and Walczak, P. Long-Term MR Imaging of Immunocompetent and Immunodeficient Mice Reveals Distinct Differences in Contrast Clearance in the Brain. Joint Annual Meeting ISMRM-ESMRMB Stockholm, Sweden, 2010 (Abstract).
Pawelczyk E, Jordan EK, Balakumaran A et al (2009) In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. Plos One 4(8):e6712
Moloney, T.C., Dockery, P., Windebank, A.J.et al. Survival and Immunogenicity of Mesenchymal Stem Cells From the Green Fluorescent Protein Transgenic Rat in the Adult Rat Brain. Neurorehabil Neural Repair.
Winter EM, Hogers B, van der Graaf LM et al (2010) Cell tracking using iron oxide fails to distinguish dead from living transplanted cells in the infarcted heart. Magn Reson Med 63(3):817–821
Jackson J, Chapon C, Jones W et al (2009) In vivo multimodal imaging of stem cell transplantation in a rodent model of Parkinson's disease. J Neurosci Methods 183(2):141–148
Sykova E, Jendelova P (2006) Magnetic resonance tracking of transplanted stem cells in rat brain and spinal cord. Neurodegener Dis 3(1–2):62–67
Dunning MD, Kettunen MI, Ffrench Constant C, Franklin RJ, Brindle KM (2006) Magnetic resonance imaging of functional Schwann cell transplants labelled with magnetic microspheres. Neuroimage 31(1):172–180
Higuchi T, Anton M, Dumler K et al (2009) Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med 50(7):1088–1094
Amsalem Y, Mardor Y, Feinberg MS et al (2007) Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 116(11 Suppl):I38–I45
Cao AH, Shi HJ, Zhang Y, Teng GJ (2009) In vivo tracking of dual-labeled mesenchymal stem cells homing into the injured common carotid artery. Anat Rec (Hoboken) 292(10):1677–1683
Lee ES, Chan J, Shuter B et al (2009) Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells 27(8):1921–1931
Jirak D, Kriz J, Strzelecki M et al (2009) Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. Magma 22(4):257–265
Dekaban GA, Snir J, Shrum B et al (2009) Semiquantitation of mouse dendritic cell migration in vivo using cellular MRI. J Immunother 32(3):240–251
Pawelczyk E, Arbab AS, Chaudhry A et al (2008) In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells 26(5):1366–1375
Acknowledgments
The authors would like to thank Elizabeth Dunn for technical assistance, Vasiliki Economopoulos for assistance with data analysis, Dr. Andrew Alejski for technical support with the MRI hardware, and Judy Sholdice and Dr. Susan Koval at the Transmission Electron Microscopy Facility in the Department of Microbiology and Immunology at The University of Western Ontario. Funding provided by the Ontario Neurotrauma Foundation (LG) and the Canadian Institutes of Health Research (PF).
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript Category and Significance: This is an original article reporting on the first study to use in vivo MRI to monitor the fate of stem cells after their direct transplantation into the injured spinal cord in mice. Our investigation indicates that caution should be taken when interpreting MR images after direct transplantation of iron-labeled cells. Cell death and subsequent phagocytosis of iron particles by macrophages may render the MRI signal nonspecific for tracking transplanted cells for long periods of time.
Rights and permissions
About this article
Cite this article
Gonzalez-Lara, L.E., Xu, X., Hofstetrova, K. et al. The Use of Cellular Magnetic Resonance Imaging to Track the Fate of Iron-Labeled Multipotent Stromal Cells after Direct Transplantation in a Mouse Model of Spinal Cord Injury. Mol Imaging Biol 13, 702–711 (2011). https://doi.org/10.1007/s11307-010-0393-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0393-y