Abstract
Purpose
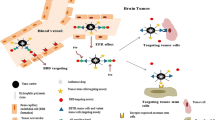
The transferrin receptor (TfR) is one of the most attractive targets to overcome the blood–brain barrier (BBB). It has recently been shown that THRPPMWSPVWP binds to the TfR and is subsequently internalized into TfR-expressing cells. Here, we evaluated the ability of THRPPMWSPVWP to become internalized into human TfR-expressing cells via endocytosis to determine its potential to act as a carrier system for the transport of small molecules across the BBB.
Procedures
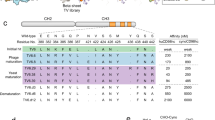
To validate the underlying concept of a conjugate consisting of a small brain imaging tracer and a large peptidic carrier molecule, a conjugate of the high affinity D2 receptor ligand fallypride and the TfR targeting peptide THRPPMWSPVWP has been synthesized. Furthermore, two derivatives of THRPPMWSPVWP were labeled with 68Ga in high radiochemical yields (>96%) and a radiochemical purity of 96–98% and evaluated in vitro and in vivo.
Results
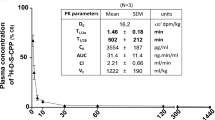
The fallypride–THRPPMWSPVWP conjugate still displayed a K i of 27 nM. The uptake of the 68Ga-labeled peptides into TfR-bearing cells was investigated using U87MG and HT-29 cells to assess the capability of the peptide to act as a carrier molecule targeting the TfR. The in vitro binding studies revealed negligible uptake of the tested 68Ga-labeled conjugates ranging from 0.08% to 0.66% after 60 min incubation at 37°C. Initial in vivo experiments with 68Ga-DOTA-S-maleimido–THRPPMWSPVWP in two healthy rats showed a mean brain uptake of 0.037% injected dose per gram, confirming the results obtained in vitro.
Conclusion
These results suggest that the accumulation of the 68Ga-radiolabeled conjugates of the TfR-binding peptide THRPPMWSPVWP into TFR expressing human cell lines is nonspecific and too low to render this peptide suitable as a possible carrier molecule for a receptor-mediated transport of compounds across the BBB.




Similar content being viewed by others
References
Schreckenberger M, Hägele S, Siessmeier T, Buchholz H-G, Armbrust-Henrich H, Rösch F, Gründer G, Bartenstein P, Vogt T (2004) The dopamine D2 receptor ligand 18F–desmethoxyfallypride: an appropriate fluorinated PET tracer for the differential diagnosis of parkinsonism. Eur J Nucl Med Mol Imag 31(8):1128–1135
Gründer G, Fellows C, Janouschek H, Veselinovic T, Boy C, Bröcheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C, Röch F, Schaefer WM, Vernaleken I (2008) Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]Fallypride PET study. Am J Psychiatry 165:988–995
Werhahn KJ, Landvogt C, Klimpe S, Buchholz HG, Yakushev I, Siessmeier T, Müller-Forell W, Piel M, Rösch F, Glaser M, Schreckenberger M, Bartenstein P (2006) Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F]Fallypride PET study. Epilepsia 47(8):1392–1396
Catafau AM, Suarez M, Bullich S, Llop J, Nucci G, Gunn RN, Brittain C, Laruelle M (2009) Within-subject comparison of striatal D2 receptor occupancy measurements using [123I]IBZM SPECT and [11C]Raclopride PET. Neuroimage 46:447–458
Reeves S, Brown R, Howard R, Grasby P (2009) Increased striatal dopamine (D2/D3) receptor availability and delusions in Alzheimer disease. Neurology 72:528–534
Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen AJ, Krasikova RN, Farde L (2009) [18F]Flumazenil binding to central benzodiazepine receptor studies by PET—quantitative analysis and comparisons with [11C]flumazenil. Neuroimage 45:891–902
Heiss W-D, Sobesky J (2008) Comparison of PET and DW/PW-MRI in acute ischemic stroke. Keio J Med 57(3):125–131
Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR (2009) Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex 19(11):2499–2507
Erritzoe D, Rasmussen H, Kristiansen KT, Frokjaer VG, Haugbol S, Pinborg L, Baaré W, Svarer C, Madsen J, Lublin H, Knudsen GM, Glenthoj BY (2008) Cortical and subcortical 5-HT2A receptor binding in neuroleptic-naive first-episode schizophrenic patients. Neuropsychopharmacology 33:2435–2441
Cilia R, Marotta G, Benti R, Pezzoli G, Antonini A (2005) Brain SPECT imaging in multiple system atrophy. J Neural Transm 112:1635–1645
Leenders KL (2003) Significance of non-presynaptic SPECT tracer methods in Parkinson’s disease. Mov Disord 18(suppl7):S39–S42
Eshuis SA, Jager PL, Maguire RP, Jonkman S, Dierck RA, Leenders KL (2009) Direct comparison of FP-CIT SPECT and F-DOPA PET in patients with Parkinson’s disease and healthy controls. Eur J Nucl Med Mol Imag 36:454–462
Qian ZM, Li H, Sun H, Ho K (2002) Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pham Rev 54(4):561–587
de Boer AG, Gaillard PJ (2007) Drug targeting to the brain. Ann Rev Pharmacol Toxicol 47:323–355
Pardridge WM (2008) Re-engineering biopharmaceuticals for delivery to brain with molecular trojan horses. Bioconjug Chem 19(7):1327–1338
Jones AR, Shusta EV (2007) Blood–brain barrier transport of therapeutics via receptor-mediation. Pharm Res 24(9):1759–1771
Calzolari A, Oliviero I, Deaglio S, Mariani G, Biffoni M, Sposi NM, Malavasi F, Peschle C, Testa U (2007) Transferrin receptor 2 is frequently expressed in human cancer cell lines. Blood Cells Mol Dis 39:82–91
Shin S-U, Friden P, Moran M, Olson T, Kang Y-S, Pardridge WM, Morrison SL (1995) Transferrin-antibody fusion proteins are effective in brain targeting. Proc Natl Acad Sci 92:2820–2824
Mishera V, Mahor S, Rawat A, Gupta PN, Dubey P, Khatri K, Vyas SP (2006) Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J Drug Target 14(1):45–53
Ulbrich K, Hekmatara T, Herbert E, Kreuter J (2009) Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur J Pharm Biopharm 71:251–256
Aktas Y, Yemisci M, Andrieux K, Gürsoy RN, Alonso MJ, Fernandez-Megia E, Novoa-Carballal R, Quinoa E, Riguera R, Sargon MF, Celik HH, Demir AS, Hıncal AA, Dalkara T, Capan Y, Couvreur P (2005) Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconj Chem 16:1503–1511
Pang Z, Lu W, Gao H, Hu K, Chen J, Zhang C, Gao X, Jiang X, Zhu C (2008) Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Contr Rel 128:120–127
Beduneau A, Saulnier P, Hindre F, Clavreul A, Leroux J-C, Benoit J-P (2007) Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab’ fragments. Biomat 28:4978–4990
Hackel BJ, Huang D, Bubolz JC, Wang XX, Shusta EV (2006) Production of soluble and active transferrin receptor-targeting single-chain antibody using Saccharomyces cerevisiae. Pharm Res 23(4):790–797
Lee JH, Engler JA, Collawn JF, Moore BA (2001) Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur J Biochem 268:2004–2012
Oh S, Kim BJ, Singh NP, Lai H, Sasaki T (2009) Synthesis and anti-cancer activity if colvalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett 274:33–39
Moos T, Morgan EH (2001) Restricted transport of anti-transferrin receptor antibody (OX26) through the blood–brain barrier in the rat. J Neurochem 79(1):119–129
Wängler C, Wängler B, Eisenhut M, Haberkorn U, Mier W (2008) Improved syntheses and applicability of different DOTA building blocks for multiply derivatized scaffolds. Bioorg Med Chem 16:2606–2616
Wängler B, Quandt G, Iovkova L, Schirrmacher E, Wängler C, Boening G, Hacker M, Schmoeckel M, Jurkschat M, Bartenstein P, Schirrmacher R (2009) Kit-like 18F-labeling of proteins: synthesis of 4-(di-tert-butyl[18F]fluorosilyl)benzenethiol (Si[18F]FA-SH) labeled rat serum albumin for blood pool imaging with PET. Bioconj Chem 20:317–321
Wellings DA, Atherton E (1997) Standard Fmoc protocols. Meth Enzymol 289:44–67
Prante O, Einsiedel J, Haubner R, Gmeiner P, Wester HJ, Kuwert T, Maschauer S (2007) 3, 4, 6-Tri-O-acetyl-2-deoxy-2-[18F]fluoroglucopyranosyl phenylthio-sulfonate: a thiol-reactive agent for the chemoselective 18F-glycosylation of peptides. Bioconjug Chem 18(1):254–262
Malmberg A, Jerning E, Mohell N (1996) Critical reevaluation of spiperone and benzamide binding to dopamine D2 receptors: evidence for identical binding sites. Eur J Pharmacol 303:123–128
Schirrmacher R, Bradtmöller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Wängler B, Niemeyer CM, Jurkschat K (2006) 18F-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew Chem Int Ed Engl 45(36):6047–6050
Schirrmacher E, Wängler B, Cypryk M, Bradtmöller G, Schäfer M, Eisenhut M, Jurkschat K, Schirrmacher R (2007) Synthesis of p-(di-tert-butyl[18F]fluorosilyl)benzaldehyde ([18F]SiFA-A) with high specific activity by isotopic exchange: a convenient labeling synthon for the 18F-labeling of N-amino-oxy derivatized peptides. Bioconjug Chem 18(6):2085–2089
Acknowledgments
This work was supported by research grants from the Natural Sciences and Engineering Council (NSERC) Canada and from the FRSQ funded Quebec Bio-Imaging Network to R. Schirrmacher.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Carmen Wängler and Dina Nada contributed equally
Ralf Schirrmacher and Olaf Prante share senior authorship
Rights and permissions
About this article
Cite this article
Wängler, C., Nada, D., Höfner, G. et al. In Vitro and Initial In Vivo Evaluation of 68Ga-Labeled Transferrin Receptor (TfR) Binding Peptides as Potential Carriers for Enhanced Drug Transport into TfR Expressing Cells. Mol Imaging Biol 13, 332–341 (2011). https://doi.org/10.1007/s11307-010-0329-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0329-6