Abstract
Introduction
Active microorganisms have been recently discovered in clouds, thus demonstrating the capacity of microorganisms to exist in harsh environments, including exposure to UV and oxidants, osmotic and cold shocks, etc. It is important to understand how microorganisms respond to and survive such stresses at the metabolic level.
Objectives
The objective of this work is to assess metabolome modulation in a strain of Pseudomonas syringae isolated from cloud water and facing temperature downshift from 17 to 5 °C by identifying key molecules and pathways of the response/adaptation to cold shock.
Methods
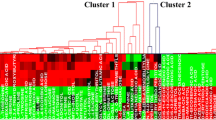
Bacterial extracts from suspensions of cells grown at 17 °C and further incubated in microcosms at 5 and 17 °C to mimic cloud conditions were analysed by combining LC-MS and NMR; the results were evaluated in comparison to similar suspensions kept at constant temperature. The differences in the metabolome profiles were deciphered using multivariate statistics (PLS-DA).
Results
Key cold shock biomarkers were observed, including cryoprotectants (trehalose, glucose, glycerol, carnitine, glutamate), antioxidants (glutathione and carnitine) and their precursors, alkaloids (bellendine and slaframine) and metabolites involved in energy metabolism (ATP, carbohydrates). Furthermore, new short peptides (nine dipeptides and a tetrapeptide) were found that have no known function.
Conclusions
This study shows that in response to cold temperatures, Pseudomonas syringae PDD-32b-74 demonstrates numerous metabolism modifications to counteract the impacts of low temperatures.



Similar content being viewed by others
References
Ahern, H. E., Walsh, K. A., Hill, T. C. J., & Moffett, B. F. (2007). Fluorescent pseudomonads isolated from Hebridean cloud and rain water produce biosurfactants but do not cause ice nucleation. Biogeosciences, 4, 115–124.
Alreshidi, M. M., Dunstan, R. H., Macdonald, M. M., Smith, N. D., Gottfries, J., & Roberts, T. K. (2015). Metabolomic and proteomic responses of Staphylococcus aureus to prolonged cold stress. Journal of Proteomics, 121, 44–55.
Amato, P., & Christner, B. C. (2009). Energy metabolism response to low-temperature and frozen conditions in Psychrobacter cryohalolentis. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.02193-08.
Amato, P., Demeer, F., Melaouhi, A., Fontanella, S., Martin-Biesse, A.-S., Sancelme, M., et al. (2007b). A fate for organic acids, formaldehyde and methanol in cloud water: Their biotransformation by microorganisms. Atmospheric Chemistry and Physics, 7, 4159–4169.
Amato, P., Joly, M., Schaupp, C., Attard, E., Moehler, O., Morris, C. E., et al. (2015). Survival and ice nucleation activity of bacteria as aerosol in a cloud simulation chamber. Atmospheric Chemistry and Physics. https://doi.org/10.5194/acp-15-1-2015.
Amato, P., Ménager, M., Sancelme, M., Laj, P., Mailhot, G., & Delort, A.-M. (2005). Microbial population in cloud water at the Puy de Dôme: Implications for the chemistry of clouds. Atmospheric Environment, 39, 4143–4153.
Amato, P., Parazols, M., Sancelme, M., Laj, P., Mailhot, G., & Delort, A.-M. (2007a). Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dôme: Major groups and growth abilities at low temperatures. FEMS Microbiology Ecology, 59, 242–254.
Amato, P., Parazols, M., Sancelme, M., Mailhot, G., Laj, P., & Delort, A.-M. (2007c). An important oceanic source of micro-organisms for cloud water at the Puy de Dôme (France). Atmospheric Environment, 41, 8253–8263.
Amezaga, M.-R., Davidson, I., McLaggan, D., Verheul, A., Abee, T., & Booth, I. R. (1995). The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology, https://doi.org/10.1099/00221287-141-1-41.
Ariya, P. A., & Amyot, M. (2004). New directions: The role of bioaerosols in atmospheric chemistry and physics. Atmospheric Environment, 38, 1231–1232.
Arvizu-Gomez, J. L., Hernadez-Morales, A., Agilar, J. R. P., & Alavrez-Morales, A. (2013). Transcriptional profile of P. syringae pv phaesicola NPS3121 at low temperature: Physiology of phytopathogenic bacteria. BMC microbiology. https://doi.org/10.1186/1471-2180-13-81.
Attard, E., Yang, H., Delort, A.-M., Amato, P., Pöschl, U., Glaux, C., et al. (2012). Effects of atmospheric conditions on ice nucleation activity of Pseudomonas. Atmospheric Chemistry and Physics, 12, 10667–10677.
Azizan, K. A., Baharum, S. N., & Mohd Noor, N. (2012). Metabolic profiling of Lactococcus lactis under different culture conditions. Molecules (Basel, Switzerland), 17, 8022–8036.
Bakermans, C., Tollaksen, S. L., Giometti, C. S., Wilkerson, C., Tiedje, J. M., & Thomashow, M. F. (2007). Proteomic analysis of Psychrobacter cryohalolentis K5 during growth at subzero temperatures. Extremophiles: Life Under Extreme Conditions. https://doi.org/10.1007/s00792-006-0042-1.
Bauer, H., Kasper-Giebl, A., Löflund, M., Giebl, H., Hitzenberger, R., Zibuschka, F., & Puxbaum, H. (2002). The contribution of bacterial and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmospheric Research, 64, 109–119.
Besaury, L., Amato, P., Sancelme, M., & Delort, A.-M. (2017). Draft genome sequence of Pseudomonas syringae PDD-32b-74, a model strain for ice-nucleation studies in the atmosphere. Genome Announcement, 5, e00742-17. https://doi.org/10.1128/genomeA.00742-17.
Bisht, S. C., Joshi, G. K., Haque, S., & Mishra, P. K. (2013). Cryotolerance strategies of Pseudomonads isolated from the rhizosphere of Himalayan plants. Springer Plus. https://doi.org/10.1186/2193-1801-2-667.
Boroujerdi, A. F., Jones, S. S., & Bearden, D. W. (2012). NMR analysis of metabolic responses to extreme conditions of the temperature-dependent coral pathogen Vibrio coralliilyticus. Letters in Applied Microbiology, 54, 209–216.
Boroujerdi, A. F., Vizcaino, M. I., Meyers, A., Pollock, E. C., Huynh, S. L., Schock, T. B., et al. (2009). NMR-based microbial metabolomics and the temperature-dependent coral pathogen Vibrio coralliilyticus. Environmental Science and Technology, 15, 7658–7664.
Caspi, R., Altman, T., Billington, R., Dreher, K., Foerster, H., Fulcher, C. A., et al. (2014). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Research, 42(D1), D459-D471.
Chamoun, R., Aliferis, K. A., & Jabaji, S. (2015). Identification of signatory secondary metabolites during mycoparasitism of Rhizoctonia solani by Stachybotrys elegans. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2015.00353.
Coucheney, E., Daniell, T. J., Chenu, C., & Nunan, N. (2008). Gas chromatographic metabolic profiling: A sensitive tool for functional microbial ecology. Journal of Microbiological Methods, 75, 491–500.
Dalmasso, M., Aubert, J., Briard-Bion, V., Chuat, V., Deutsch, S.-M., Even, S., et al. (2012). A Temporal -omic Study of Propionibacterium freudenreichii CIRM-BIA1T adaptation strategies in conditions mimicking cheese ripening in the cold. PLoS ONE. https://doi.org/10.1371/journal.pone.0029083.
Davies, K. J. A. (2000). Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life, 50, 279–289.
Degrassi, G., Aguilar, C., Bosco, M., Zahariev, S., Pongor, S., & Venturi, V. (2002). Plant growth-promoting Pseudomonas putida WCS358 produces and secretes cyclid dipetides: Cross talk with quorum sensing bacterial sensors. Current Microbiology, 45, 250–254.
Deguillaume, L., Charbouillot, T., Joly, M., Vaïtilingom, M., Parazols, M., Marinoni, A., et al. (2014). Classification of clouds sampled at the puy de Dôme (France) based on 10 year of monitoring of their physicochemical properties. Atmospheric Chemistry and Physics, 14, 1485–1506.
Delort, A.-M., Vaïtilingom, M., Amato, P., Sancelme, M., Parazols, M., Laj, P., et al. (2010). A short overview of the microbial population in clouds: potential roles in atmospheric chemistry and nucleation processes. Atmospheric Research, 98, 249–260.
Després, V. R., Huffman, J. A., Burrows, S. M., Hoose, C., Safatov, A. S., Buryak, G., et al. (2012). Primary biological particles in the atmosphere: a review. Tellus B, 64, 1–58.
Fajardo, V. A., McMeekin, L., & LeBlanc, P. J. (2011). Influence of phospholipid species on membrane fluidity: A meta-analysis for a Novel Phospholipid Fluidity Index. The Journal of Membrane Biology, 244, 97–110.
Fan, W.-M. (1996). Metabolite profiling by one-and two-dimensional NMR analysis of complex mixtures. Progress in Nuclear Magnetic Resonance Spectroscopy, 28, 161–219.
Feller, G., & Gerday, C. (2003). Psychrophilic enzymes: Hot topics in cold adaptation. Nature Reviews Microbiology. https://doi.org/10.1038/nrmicro773.
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., et al. (2016). Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmospheric Research, 182, 346–376.
Hill, K. A., Shepson, P. B., Galbavy, E. S., Anastasio, C., Kourtev, P. S., Konopka, A., & Stirm, B. H. (2007). Processing of atmospheric nitrogen by clouds above a forest environment. Journal of Geophysical Research. https://doi.org/10.1029/2006JD008002.
Joly, M., Amato, P., Sancelme, M., Vinatier, V., Abrantes, M., Deguillaume, L., & Delort, A.-M. (2015). Survival of microbial isolates from clouds toward simulated atmospheric stress factors. Atmospheric Environment. https://doi.org/10.1016/j.atmosenv.2015.07.009.
Joly, M., Attard, E., Sancelme, M., Deguillaume, L., Guilbaud, C., Morris, C. E., et al. (2013). Ice nucleation activity of bacteria isolated from cloud water. Atmospheric Environment, 70, 392–400.
Jozefczuk, S., Klie, S., Catchpole, G., Szymanski, J., Cuadros-Inostroza, A., Steinhauser, D., et al. (2010). Metabolomic and transcriptomic stress response of Escherichia coli. Molecular Systems Biology. https://doi.org/10.1038/msb.2010.18.
Kanehisa, M., & Goto, S. (2000). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research, 28, 27–30.
Kol, S., Merlo, M. E., Scheltema, R. A., de Vries, M., Vonk, R. J., Kikkert, N. A., et al. (2010). Metabolomic characterization of the salt stress response in Streptomyces coelicolor. Applied and Environmental Microbiology, 76, 2574–2581.
Kourtev, P. S., Hill, K. A., Shepson, P. B., & Konopka, A. (2011). Atmospheric cloud water contains a diverse bacterial community. Atmospheric Environment, 45, 5399–5405.
Kuhl, C., Tautenhahn, R., Böttcher, C., Larson, T. R., & Neumann, S. (2012). CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Analytical Chemistry, 84(1), 283–289.
Kurz, M., Burch, Y. A., Seip, B., Lindow, S. E., & Gross, H. (2010). Genome driven investigation of compatible solute biosynthesis pathway of Pseudomonas syringae pv syringae and their contribution to water stress tolerance. Applied and Environmental Microbiology, 76, 5452–5462.
Le Marrec, C., Bon, E., & Lonvaud-Funel, A. (2007). Tolerance to high osmolality of the lactic acid bacterium Oenococcus oeni and identification of potential osmoprotectants. International Journal of Food Microbiology, 115, 335–342.
Lin, C. Y., Wu, H., Tjeerdema, R. S., & Viant, M. R. (2007). Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics, 3, 55–67.
Miranda, H., Cheregi, O., Netotea, S., Hvidsten, T. R., Moritz, T., & Funk, C. (2013). Co-expression analysis, proteomic and metabolomic study on the impact of a Deg/HtrA protease triple mutant in Synechocystis sp. PCC 6803 exposed to temperature and high light stress. Journal of Proteomics, 78, 294–311.
Nicholson, J. K., Foxall, P. J., Spraul, M., Farrant, R. D., & Lindon, J. C. (1995). 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Analytical Chemistry, 67(5), 793–811.
Piuri, M., Sanchez-Rivas, C., & Ruzal, S. M. (2003). Adaptation to high salt in Lactobacillus: role of peptides and proteolytic enzymes. Journal of Applied Microbiology, 95, 372–379.
Rabinowitz, J. D., & Kimball, E. (2007). Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Analytical Chemistry, 79, 6167–6173.
Renard, P., Canet, I., Sancelme, M., Wirgot, N., Deguillaume, L., & Delort, A.-M. (2016). Screening of cloud microorganisms isolated at the Puy de Dôme (France) station for the production of biosurfactants. Atmospheric Chemistry and Physics. https://doi.org/10.5194/acp-16-12347-2016.
Sagot, B., Gaysinski, M., Mehiri, M., Guigonis, J. M., Le Rudulier, D., & Alloing, G. (2010). Osmotically induced synthesis of the dipeptide N-acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria. Proceedings of the National Academy of Sciences, https://doi.org/10.1073/pnas.1003063107.
Šantl-Temkiv, T., Finster, K., Dittmar, T., Hansen, B. M., Thyrhaug, R., Nielsen, N. W., Karlson, U. G. (2013). Hailstones: A Window into the Microbial and Chemical Inventory of a Storm Cloud. PLoS ONE. https://doi.org/10.1371/journal.pone.0053550.
Sardesai, N., & Babu, C. R. (2000). Cold stress induces switchover of respiratory pathway to lactate glycolysis in psychrotrophic Rhizobium strains. Folia Microbiologica. https://doi.org/10.1007/BF02817420.
Sattler, B., Puxbaum, H., & Psenner, R. (2001). Bacterial growth in supercooled cloud droplets. Geophysical Research Letters, 28, 239–242.
Shin, M. H., Lee, D. Y., Liu, K.-H., Fiehn, O., & Kim, K. H. (2010). Evaluation of sampling and extraction methodologies for the global metabolic profiling of Saccharophagus degradans. Analytical Chemistry, 82, 6660–6666.
Shivaji, S., & Prakash, J. S. (2010). How do bacteria sense and respond to low temperature? Archives of Microbiology, 192, 85–95.
Singh, A. K., Ulanov, A. V., Li, Z., Jayaswal, R. K., & Wilkinson, B. J. (2011). Metabolomes of the psychrotolerant bacterium Listeria monocytogenes 10403S grown at 37 °C and 8 °C. International Journal of Food Microbiology, 148, 107–114.
Smith, C. A., O’Maille, G., Want, E. J., Qin, C., Trauger, S. A., Brandon, T. R., et al. (2005). METLIN: A metabolite mass spectral database. Therapeutic Drug Monitoring, 27(6), 747–751.
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R., & Siuzdak, G. (2006). XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Analytical Chemistry, 78, 779–787.
Smith, L. T., & Smith, G. M. (1989). An osmoregulated dipeptide in stressed Rhizobium meliloti. Journal of Bacteriology. https://doi.org/10.1128/jb.171.9.4714-4717.1989.
Sumner, L. W., Amberg, A., Barrett, D., Beale, M. H., Beger, R., Daykin, C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3, 211–221.
Sumner, L. W., Lei, Z., Nikolau, B. J., Saito, K., Roessner, U., & Trengove, R. (2014). Proposed quantitative and alphanumeric metabolite identification metrics. Metabolomics, 10, 1047–1049.
Vaïtilingom, M., Attard, E., Gaiani, N., Sancelme, M., Deguillaume, L., Flossmann, A. I., et al. (2012). Long-term features of cloud microbiology at the puy de Dôme (France). Atmospheric Environment, 56, 88–100.
Vaïtilingom, M., Charbouillot, T., Deguillaume, L., Maisonobe, R., Parazols, M., Amato, et al. (2011). Atmospheric chemistry of carboxylic acids: Microbial implication versus photochemistry. Atmospheric Chemistry and Physics, 11, 8721–8733.
Vaïtilingom, M., Deguillaume, L., Vinatier, V., Sancelme, M., Amato, P., Chaumerliac, N., & Delort, A.-M. (2013). Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds. Proceedings of the National Academy of Sciences, 110(2), 559564.
Welsh, D. T. (2000). Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiology Reviews, https://doi.org/10.1111/j.1574-6976.2000.tb00542.x.
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2013). HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Research, 41(D1), D801-D807.
Ye, Y., Zhang, L., Hao, F., Zhang, J., Wang, Y., & Tang, H. (2012). Global metabolomic responses of Escherichia coli to heat stress. Journal of Proteome Research, 11, 2559–2566.
Zaprasis, A., Brill, J., Thüring, M., Wünsche, G., Heun, M., Barzantny, H., et al. (2013). Osmoprotection of Bacillus subtilis through import and proteolysis of pro-line-containing peptides. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.01934-12.
Zhang, J., Li, Y., Chen, W., Du, G. C., & Chen, J. (2012). Glutathione improves the cold resistance of Lacotococcus sanfranciscensis by physiological regulation. Food Microbiology, 31, 285–292.
Acknowledgements
This research has been supported by the ANR French program MetaboHUB. We would like to thank Mélanie Pétéra for her valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Research involving with human and animal participants
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jousse, C., Dalle, C., Canet, I. et al. Metabolomic study of the response to cold shock in a strain of Pseudomonas syringae isolated from cloud water. Metabolomics 14, 11 (2018). https://doi.org/10.1007/s11306-017-1295-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1295-7