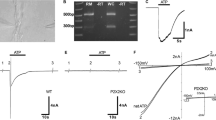
Abstract
Gap junction-mediated K+ recycling in the cochlear supporting cell has been proposed to play a critical role in hearing. However, how potassium ions enter into the supporting cells to recycle K+ remains undetermined. In this paper, we report that ATP can mediate K+ sinking to recycle K+ in the cochlear supporting cells. We found that micromolar or submicromolar levels of ATP could evoke a K+-dependent inward current in the cochlear supporting cells. At negative membrane potentials and the resting membrane potential of −80 mV, the amplitude of the ATP-evoked inward current demonstrated a linear relationship to the extracellular concentration of K+, increasing as the extracellular concentration of K+ increased. The inward current also increased as the concentration of ATP was increased. In the absence of ATP, there was no evoked inward current for extracellular K+ challenge in the cochlear supporting cells. The ATP-evoked inward current could be inhibited by ionotropic purinergic (P2X) receptor antagonists. Application of pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS, 50 µM) or pre-incubation with an irreversible P2X7 antagonist oxidized ATP (oATP, 0.1 mM) completely abolished the ATP-evoked inward current at the negative membrane potential. ATP also evoked an inward current at cell depolarization, which could be inhibited by intracellular Cs+ and eliminated by positive holding potentials. Our data indicate that ATP can activate P2X receptors to recycle K+ in the cochlear supporting cells at the resting membrane potential under normal physiological and pathological conditions. This ATP-mediated K+ recycling may play an important role in the maintenance of cochlear ionic homeostasis.









Similar content being viewed by others
References
Zhao HB, Kikuchi T, Ngezahayo A, White TW (2006) Gap junctions and cochlear homeostasis. J Membr Biol 209:177–186
Mistrik P, Ashmore J (2009) The role of potassium recirculation in cochlear amplification. Curr Opin Otolaryngol Head Neck Surg 17:394–399
Santos-Sacchi J, Dallos P (1983) Intercellular communication in the supporting cells of the organ of Corti. Hear Res 9:317–326
Santos-Sacchi J (1991) Isolated supporting cells from the organ of Corti: some whole cell electrical characteristics and estimates of gap junctional conductance. Hear Res 52:89–98
Kikuchi T, Kimura RS, Paul DL, Adams JC (1995) Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol 191:101–118
Spicer SS, Schulte BA (1998) Evidence for a medial K+ recycling pathway from inner hair cells. Hear Res 118:1–12
Zhao HB, Santos-Sacchi J (1998) Effect of membrane tension on gap junctional conductance of supporting cells in Corti’s organ. J Gen Physiol 112:447–455
Zhao HB, Santos-Sacchi J (2000) Voltage gating of gap junctions in cochlear supporting cells: evidence for nonhomotypic channels. J Membr Biol 175:17–24
Zhao HB (2000) Directional rectification of gap junctional voltage gating between dieters cells in the inner ear of guinea pig. Neurosci Lett 296:105–108
Ashmore JF, Ohmori H (1990) Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol 428:109–131
Nakagawa T, Akaike N, Kimitsuki T, Komune S, Arima T (1990) ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol 63:1068–1074
Dulon D, Mollard P, Aran JM (1991) Extracellular ATP elevates cytosolic Ca2+ in cochlear inner hair cells. NeuroReport 2:69–72
Sugasawa M, Erostegui C, Blanchet C, Dulon D (1996) ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol 491:707–718
Jacobson KA, Jarvis MF, Williams M (2002) Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 45:4057–4093
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Housley GD, Greenwood D, Ashmore JF (1992) Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc R Soc Lond B 249:265–273
Housley GD, Luo L, Ryan AF (1998) Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol 393:403–414
Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR (1999) Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci 19:8377–8388
Chen C, Bobbin RP (1998) P2X receptors in cochlear Deiters’ cells. Br J Pharmacol 124:337–344
Jarlebark LE, Housley GD, Thorne PR (2000) Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X2 receptor subunits in adult and developing rat cochlea. J Comp Neurol 421:289–301
Jarlebark L, Housley GD, Raybould NP, Vlajkovic S, Thorne PR (2002) ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea. NeuroReport 13:1979–1984
Parker MS, Nkeiruka NO, Bobbin RP (2003) Localization of the P2Y4 receptor in the guinea pig organ of Corti. J Am Acad Audiol 14:286–295
Szucs A, Szappanos H, Toth A, Farkas Z, Panyi G, Csernoch L, Sziklai I (2004) Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear Res 196:2–7
Zhao HB, Yu N, Fleming CR (2005) Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA 102:18724–18729
Yu N, Zhao HB (2008) ATP activates P2x receptors and requires extracellular Ca++ participation to modify outer hair cell nonlinear capacitance. Pflugers Arch 457:453–461
Zhao HB (2005) Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signaling and metabolic communications. Eur J Neurosci 21:1859–1868
Munoz DJ, Thorne PR, Housley GD, Billett TE (1995) Adenosine 5′-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear Res 90:119–125
Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105:18770–18775
Nikolic P, Housley GD, Thorne PR (2003) Expression of the P2X7 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the developing and adult rat cochlea. Audiol Neurootol 8:28–37
Ji N, Zhao HB (2005) Expressions of ATP-gated purinergic (P2) receptors in the cochlear outer hair cells. The 28th Association for Research in Otolaryngology Annual Meeting, New Orleans, LA, 19–24 February. Available at http://www.aro.org
Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537
Zhou Z, Hume RI (1998) Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. J Physiol 507:353–364
Fujiwara Y, Keceli B, Nakajo K, Kubo Y (2009) Voltage- and [ATP]-dependent gating of the P2X(2) ATP receptor channel. J Gen Physiol 133:93–109
Raybould NP, Jagger DJ, Housley GD (2001) Positional analysis of guinea pig inner hair cell membrane conductances: implications for regulation of the membrane filter. J Assoc Res Otolaryngol 2:362–376
Gossman DG, Zhao HB (2008) Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes 15:305–315
Wang XH, Streeter M, Liu YP, Zhao HB (2009) Identification and characterization of pannexin expression in the mammalian cochlea. J Comp Neurol 512:336–546
Shestopalov VI, Panchin Y (2008) Pannexins and gap junction protein diversity. Cell Mol Life Sci 65:376–394
Munoz DJ, Kendrick IS, Rassam M, Thorne PR (2001) Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol 121:10–15
Johnstone BM, Patuzzi R, Syka J, Syková E (1989) Stimulus-related potassium changes in the organ of Corti of guinea-pig. J Physiol 408:77–92
Yu N, Zhao HB (2009) Modulation of outer hair cell electromotility by cochlear supporting cells and gap junctions. PLoS ONE 4:e7923
Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G (2003) Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol 467:207–231
Zhao HB, Yu N (2006) Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol 499:506–518
Liu YP, Zhao HB (2008) Cellular characterization of Connexin26 and Connnexin30 expression in the cochlear lateral wall. Cell Tissue Res 333:395–403
Housley GD, Raybould NP, Thorne PR (1998) Fluorescence imaging of Na+ influx via P2X receptors in cochlear hair cells. Hear Res 119:1–13
Lee JH, Chiba T, Marcus DC (2001) P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci 21:9168–9174
Housley GD, Bringmann A, Reichenbach A (2009) Purinergic signaling in special senses. Trends Neurosci 32:128–141
Zhao HB (2003) Biophysical properties and functional analysis of inner ear gap junctions for deafness mechanisms of nonsyndromic hearing loss. Proceedings of the 9th International Meeting on Gap Junctions, Cambridge, UK, August 23–28
Zhao HB (2005) What is the function of connexin 26 in the cochlea? Potassium recycling or intercellular signaling and nutrient/energy supplies? In: Lim DJ (ed) Meniere’s disease and inner ear homeostasis disorders. Proceedings of the 5th International Symposium. April, 2005, Los Angeles, CA, pp 254–255
Acknowledgments
This work was supported by NIDCD DC 05989.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
(DOC 190 kb)
Supplemental Fig. S2
(DOC 87 kb)
Supplemental Fig. S3
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhao, HB. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signalling 6, 221–229 (2010). https://doi.org/10.1007/s11302-010-9184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-010-9184-9