Abstract
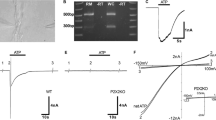
Intracochlear ATP is an important mediator in regulating hearing function. ATP can activate ionotropic purinergic (P2x) and metabotropic purinergic (P2y) receptors to influence cell functions. In this paper, we report that ATP can activate P2x receptors directly to modify outer hair cell (OHC) electromotility, which is an active cochlear amplifier determining hearing sensitivity and frequency selectivity in mammals. We found that ATP, but not UTP, a P2y receptor agonist, reduced the OHC electromotility-associated nonlinear capacitance (NLC) and shifted its voltage dependence to the right (depolarizing) direction. Blockage of the activation of P2x receptors by pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), suramin, and 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS) could block the ATP effect. This modification also required extracellular Ca++ participation. Removal of extracellular Ca++ abolished the ATP effect. However, chelation of intracellular Ca++ concentration by a fast calcium-chelating reagent 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA, 10 mM) did not affect the effect of ATP on NLC. The effect is also independent of K+ ions. Substitution of Cs+ for intracellular or extracellular K+ did not affect the ATP effect. Our findings indicate that ATP activates P2x receptors instead of P2y receptors to modify OHC electromotility. Extracellular Ca++ is required for this modification.








Similar content being viewed by others
References
Bobbin RP, Thompson MH (1978) Effects of putative transmitters on afferent cochlear transmission. Ann Otol Rhinol Larygnol 87:185–190
Kujawa SG, Erostegui C, Fallon M, Crist J, Bobbin RP (1994) Effects of adenosine 5′-triphosphate and related agonists on cochlear function. Hear Res 76:87–100
Munoz DJ, Thorne PR, Housley GD, Billett TE, Battersby JM (1995) Extracellular adenosine 5′-triphosphate (ATP) in the endolymphatic compartment influences cochlear function. Hear Res 90:106–118
Munoz DJ, Thorne PR, Housley GD (1999) P2X receptor-mediated changes in cochlear potentials arising from exogenous adenosine 5′-triphosphate in endolymph. Hear Res 138:56–64
Sueta T, Paki B, Everett AW, Robertson D (2003) Purinergic receptors in auditory neurotransmission. Hear Res 183:97–108
Ashmore JF, Ohmori H (1990) Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol 428:109–131
Nakagawa T, Akaike N, Kimitsuki T, Komune S, Arima T (1990) ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol 63:1068–1074
Dulon D, Mollard P, Aran JM (1991) Extracellular ATP elevates cytosolic Ca2+ in cochlear inner hair cells. NeuroReport 2:69–72
Housley GD, Greenwood D, Ashmore JF (1992) Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc R Soc Lond B 249:265–273
Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR (1999) Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci 19:8377–8388
Sugasawa M, Erostegui C, Blanchet C, Dulon D (1996) ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol 491:707–718
Skellett RA, Chen C, Fallon M, Nenov AP, Bobbin RP (1997) Pharmacological evidence that endogenous ATP modulates cochlear mechanics. Hear Res 111:42–54
Brownell WE, Bader CR, Bertrand D, Ribaupierre Y (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science 227:194–196
Dallos P (1992) The active cochlea. J Neurosci 12:4575–4585
Zhao HB, Santos-Sacchi J (1999) Auditory collusion and a coupled couple of outer hair cells. Nature 399:359–362
Zhao HB, Yu N, Fleming CR (2005) Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A 102:18724–18729
Jacobson KA, Jarvis MF, Williams M (2002) Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 45:4057–4093
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Raybould NP, Housley GD (1997) Variation in expression of the outer hair cell P2X receptor conductance along the guinea-pig cochlea. J Physiol 498:717–727
Brandle U, Zenner HP, Ruppersberg JP (1999) Gene expression of P2x7-receptors in the developing inner ear of the rat. Neurosci Lett 273:105–108
Jarlebark L, Housley GD, Raybould NP, Vlajkovic S, Thorne PR (2002) ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea. NeuroReport 13:1979–1984
Jarlebark LE, Housley GD, Thorne PR (2000) Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X2 receptor subunits in adult and developing rat cochlea. J Comp Neurol 421:289–301
Szucs A, Szappanos H, Toth A, Farkas Z, Panyi G, Csernoch L, Sziklai I (2004) Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear Res 196:2–7
Parker MS, Nkeiruka NO, Bobbin RP (2003) Localization of the P2Y4 receptor in the guinea pig organ of Corti. J Am Acad Audiol 14:286–295
Yu N, Zhao HB (2005) Extracellular ATP mediates outer hair cell electromotility. Proceedings of the 28th Association for Research in Otolaryngology Annual Meeting, New Orleans, LA, 19–24 February. Available at http://www.aro.org
Yu N, Zhu ML, Zhao HB (2006) Prestin is expressed on the whole outer hair cell basolateral surface. Brain Res 1095:51–58
Santos-Sacchi J, Zhao HB (2003) Excitation of fluorescent dyes inactivates the outer hair cell integral membrane motor protein prestin and betrays its lateral mobility. Pflugers Arch 446:617–622
Santos-Sacchi J, Kakehata S, Takahashi S (1998) Effects of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J Physiol 510:225–235
Munoz DJ, Thorne PR, Housley GD, Billett TE (1995) Adenosine 5′-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear Res 90:119–125
Ji N, Zhao HB (2005) Expressions of ATP-gated purinergic (P2) receptors in the cochlear outer hair cells. Proceedings of the 28th Association for Research in Otolaryngology Annual Meeting, New Orleans, LA, 19–24 February. Available at http://www.aro.org
Bobbin RP, Salt AN (2005) ATP-gamma-S shifts the operating point of outer hair cell transduction towards scala tympani. Hear Res 205:35–43
Dallos P, He DZZ, Sziklai I, Metha S, Evans BN (1997) Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci 17:2212–2226
Frolenkov GI, Mammano F, Belyantseva IA, Coling D, Kachar B (2000) Two distinct Ca2+-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci 20:5940–5948
Housley GD, Marcotti W, Navaratnam D, Yamoah EN (2006) Hair cells—beyond the transducer. J Membr Biol 209:89–118
Mammano F, Frolenkov GI, Lagostena L, Belyantseva IA, Kurc M, Dodane V, Colavita A, Kachar B (1999) ATP-induced Ca2+ release in cochlear outer hair cells: localization of an inositol triphosphate-gated Ca2+ store to the base of the sensory hair bundle. J Neurosci 19:6918–6929
Blanchet C, Erostegui C, Sugasawa M, Dulon D (1996) Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci 16:2574–2584
Evans MG (1996) Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol 491:563–578
Yu N, Zhao HB (2008) Cytoskeleton mediates outer hair cell electromotility and memory function. Proceedings of the 31st Association for Research in Otolaryngology Annual Meeting, Phoenix, AZ, 16–21 February. Available at http://www.aro.org
Zhao HB (2005) Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signaling and metabolic communications. Eur J Neurosci 21:1859–1868
Fridberger A, Flock A, Ulfendahl M, Flock B (1998) Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci U S A 95:7127–7132
Acknowledgements
This work was supported by NIDCD DC 05989. We thank P.G. Wilson for the technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, N., Zhao, HB. ATP activates P2x receptors and requires extracellular Ca++ participation to modify outer hair cell nonlinear capacitance. Pflugers Arch - Eur J Physiol 457, 453–461 (2008). https://doi.org/10.1007/s00424-008-0522-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-008-0522-5