Abstract
Secretomotor reflexes in the gastrointestinal (GI) tract are important in the lubrication and movement of digested products, absorption of nutrients, or the diarrhea that occurs in diseases to flush out unwanted microbes. Mechanical or chemical stimulation of mucosal sensory enterochromaffin (EC) cells triggers release of serotonin (5-HT) (among other mediators) and initiates local reflexes by activating intrinsic primary afferent neurons of the submucous plexus. Signals are conveyed to interneurons or secretomotor neurons to stimulate chloride and fluid secretion. Inputs from myenteric neurons modulate secretory rates and reflexes, and special neural circuits exist to coordinate secretion with motility. Cellular components of secretomotor reflexes variably express purinergic receptors for adenosine (A1, A2a, A2b, or A3 receptors) or the nucleotides adenosine 5′-triphosphate (ATP), adenosine diphosphate (ADP), uridine 5′-triphosphate (UTP), or uridine diphosphate (UDP) (P2X1-7, P2Y2, P2Y4, P2Y6, P2Y12 receptors). This review focuses on the emerging concepts in our understanding of purinergic regulation at these receptors, and in particular of mechanosensory reflexes. Purinergic inhibitory (A1, A3, P2Y12) or excitatory (A2, P2Y1) receptors modulate mechanosensitive 5-HT release. Excitatory (P2Y1, other P2Y, P2X) or inhibitory (A1, A3) receptors are involved in mechanically evoked secretory reflexes or “neurogenic diarrhea.” Distinct neural (pre- or postsynaptic) and non-neural distribution profiles of P2X2, P2X3, P2X5, P2Y1, P2Y2, P2Y4, P2Y6, or P2Y12 receptors, and for some their effects on neurotransmission, suggests their role in GI secretomotor function. Luminal A2b, P2Y2, P2Y4, and P2Y6 receptors are involved in fluid and Cl-, HCO3 -, K+, or mucin secretion. Abnormal receptor expression in GI diseases may be of clinical relevance. Adenosine A2a or A3 receptors are emerging as therapeutic targets in inflammatory bowel diseases (IBD) and gastroprotection; they can also prevent purinergic receptor abnormalities and diarrhea. Purines are emerging as fundamental regulators of enteric secretomotor reflexes in health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal (GI) secretions in coordination with motility are important in digestion of food particles, lubrication, nutrient absorption, regulation of pH and solute concentration, and elimination of waste products. Diarrhea occurs in GI diseases to flush out noxious chemicals or unwanted microbes. Intestinal chloride secretion provides a driving force for fluid movement and obligate transport of water into the lumen. Under normal circumstances, mucosal chloride secretion is very low, but can reach extraordinary rates when challenged by enterotoxins or various secretogogues. Hallmarks of enteric infections or inflammatory bowel diseases (IBD) are malaise and diarrhea; the other extreme is constipation that occurs in patients with idiopathic constipation, constipation-predominant irritable bowel syndrome (IBS), or opioid-induced constipation. Structural or functional changes in the enteric nervous system (ENS), in neurotransmitters, signaling pathways, or other components of enteric neural reflexes may be the basis for the pathogenesis of disturbances in gut motor function.
The current review will focus primarily on the role of purines in secretomotor function in the GI system. The enterochromaffin–neural circuit–epithelial reflex pathway is our target for understanding secretomotor function in the intestinal tract. All cellular components of mucosal reflexes express purinergic receptors, and this review will focus on these receptors, their distribution, and function in mucosal reflexes and secretomotor function in the GI tract. The enteric neural circuits and cellular components involved in neurosecretory reflexes will first be briefly reviewed. A brief overview and basic knowledge of purinoceptor classification, pharmacology, receptor ligands and their selectivity is necessary in order to understand the concepts forwarded in this review. Emerging concepts will be reviewed in our understanding of purinergic regulation in mucosal reflexes, with particular emphasis on enterochromaffin cells (EC), mucosal reflexes, receptor distribution, and luminal P2 receptors involved in epithelial ion transport and fluid secretion. In addition, we review recent findings on purinergic regulation of gastric secretions, and in relation to gastric mucosal protection. Our review would not be complete without a very brief description of the emerging role of purinergic receptors as therapeutic targets in IBD [2] or implications of purine receptor abnormalities (up- or downregulation) in GI diarrhea diseases. The reader is also referred to more general reviews on purinergic signaling [12], GI secretomotor function and dysfunction [25, 26], epithelial secretion, and hepatobiliary function [92]. Other relevant reviews include adenosine neuromodulation [20], purines in mechanosensory signaling [26], ATP as a sensory transmitter [6], and P2Y1 receptors in secretomotor function [113], or P2X receptors as potential targets in IBS [40].
Enterochromaffin–neural–epithelial reflex pathways
The secretory reflex is composed of sensory EC cells, intrinsic primary afferent neurons (IPANs), various interneurons, secretomotor neurons, interplexus neurons for coordinating secretion and motility or for secretomotor function [11], and the epithelial cells involved in ion transport and fluid secretion in the gut lumen (Fig. 1). Luminal stimulation by nutrients, changes in pH, mechanical forces, changes in solute concentration, luminal irritants, or invading enteropathogenic microorganisms will activate the EC cells to secrete serotonin (5-HT) among other mediators to trigger secretomotor reflexes leading to chloride, bicarbonate, potassium, mucin, and fluid secretion. EC cells communicate with both submucosal and myenteric IPANS to initiate secretomotor reflexes. Mucosal stimulation and activation of secretomotor reflexes via long myenteric pathways may play a role in the coordination of intestinal motility, secretion, and blood flow [88]. This review is restricted to submucous pathways and secretomotor reflexes. Under physiological circumstances, large fluctuations in secretions can occur depending on the stimulus. Crohn’s disease or Clostridium difficile toxin A (TxA) can cause diarrhea and alterations in secretion. However, the diarrhea observed in IBD is mainly malabsorptive not secretory; in the inflamed gut, secretion actually decreases. Hypersecretion due to infection is helpful in flushing to rid the lumen of a pathogen.
Generic intestinal secretory reflex based on guinea pig intestine. Neural reflex pathway regulating secretion includes EC sensory cells that release 5-HT to activate IPANS projecting to the mucosa, to submucous ganglia, and to myenteric ganglia. IPANS synapse with several types of cholinergic (CHAT) and vasoactive intestinal peptide (VIP) secretomotor neurons and release acetylcholine (ACh) at muscarinic receptors (M3) and VIP at VIP receptors (VPACRs) on epithelial cells, or arterioles. VIP neurons are subdivided according to their ability to generate cAMP as VIP/cAMP (+) and VIP/cAMP (-) and may be further subdivided into VIP/cAMP/A2aRs and VIP/cAMP/A2aR (-). Cholinergic neurons are ChAT/calretinin (CALRET), CHAT/NPY, CHAT (-). Mechanical distortion such as brush stroking the mucosa is one stimulus for activation of the reflex. Myenteric secretomotor neurons project to the mucosa as well. CGRP calcitonin gene-related peptide, EC enterochromaffin cells, 5-HT 5-hydroxytryptamine, NK1 neurokinin-1, NPY neuropeptide Y, SP substance P, A2aRs adenosine A2a receptors. (Modified from [25] by permission)
Release of sensory mediators from EC cells activates the afferent nerve endings of IPANs to trigger a secretory reflex. IPANs detect sensory information from the luminal environment in the form of action potentials carried in their primary afferent process. The sensory information is decoded and integrated in the cell soma and then relayed to interneurons or secretomotor neurons to elicit a secretory reflex. These IPANs in submucous ganglia have AH cell electrophysiological characteristics and comprise 15% of submucous neurons (and 25-30% of myenteric neurons). The axonal processes of submucous IPANs are known to project to other IPANs and to submucous or myenteric neurons. They have Dogiel type II morphology and project in all directions in the submucous plexus [25].
One type of IPAN in the submucous plexus has one sensory process projecting to the mucosa and responds to mucosal distortion/deformation at sites less than 1 mm2 from the recording site. Another type of IPAN in the submucous plexus is sensitive to distention [26, 38, 112]. Notable differences exist in the chemical coding of these neurons in human, porcine, and rodent intestine [25]. After activation of IPANs, release of transmitters at synapses with interneurons or secretomotor neurons leads to epithelial secretion. 5-HT interplexus neurons send a process to synapse with secretomotor neurons expressing 5-HT3 receptors. Secretomotor neurons are S/type 1 neurons that release acetylcholine or VIP to activate the chloride-secreting crypt epithelial cells expressing muscarinic and VIP receptors. All cellular components of this reflex have functional adenosine (P1) or nucleotide (P2X and P2Y) receptor subtypes and are the subject of this review.
Purinergic receptors
Adenosine, adenosine 5′-triphosphate (ATP), ADP, AMP, uridine 5′-triphosphate (UTP), UDP, and UDP-glucose are endogenous purines that activate P1, P2X, or P2Y purinoceptor families that are widely and differentially distributed in the ENS and non-neuronal cells in the GI tract. The tissue distribution and/or biological experiments suggest that up to 14 of 18 purinoceptors may be involved in secretomotor reflexes in the GI tract. Still, our knowledge remains incomplete.
Adenosine interacts with four cell surface P1 receptors, designated as A1, A2a, A2b, and A3 receptors to influence a variety of physiological functions. The endogenous ligand is adenosine (or AMP, [41]). Receptor classification is based on receptor cloning, pharmacological studies, and mouse receptor knockout models. All four receptors are expressed in the GI tract, but their characterization is incomplete (see later). Adenosine receptors belong to the superfamily of seven transmembrane domain G protein-coupled receptors (GPCR) and they are emerging as potential therapeutic targets [53].
The nomenclature for the transmitter-gated ion channel P2X1-7 receptors relies mostly on recombinant receptor pharmacology as reviewed by Khakh et al. [57]; the pharmacology of the P2X receptors in whole tissues is complicated by species differences, marked ectonucleotidase activity that interferes with agonist/antagonist potency profiles, and lack of very selective ligands for P2X receptor subtypes. Their review covers the functional properties of recombinant receptors, their pharmacology, ion effects (Zn2+, H+, Ca2+), and some native receptors in tissues.
A recent review summarizes the pharmacological profiles of cloned mammalian metabotropic P2Y receptors [110] that have been shown to mediate actions of nucleotides when expressed in functional systems. Eight P2Y receptors have been cloned and defined functionally. The P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors are coupled to stimulation of phospholipase C (PLC). P2Y12, P2Y13, and P2Y14 receptors are negatively coupled to adenylyl cyclase (AC); the P2Y11 receptor is positively coupled to AC. Abbrachio et al. [1] provide an update on molecular mechanisms, pathophysiology, and therapeutics of P2Y receptors.
P2Y1 receptors are involved in platelet aggregation, vasodilation, neuromodulation, mechanosensitivity, and secretomotor reflexes. The P2Y1 receptor is activated by ADP or its analog 2-methylthioADP (2MeSADP). A potent and selective antagonist is 2′-deoxy-N6-methyladenosine-3′5′-bisphosphate (MRS2179) or MRS2279. Adenosine 5′-O-(1-boranotriphosphate) (ATP-α-B) derivatives are novel P2Y1 receptor agonists that may be of potential clinical relevance [78].
The P2Y2 receptor plays an important role in Cl- secretion from epithelial cells, and a P2Y2 receptor agonist diquafosol is used for treatment of dry eye disease. It is activated by UTP and ATP and their actions are blocked by suramin, although suramin has affinity for non-purinergic receptors as well [54]. The P2Y4 receptor is expressed in epithelia, and depending on the species, the receptor has a strong preference for UTP (human receptor) or equal preference for UTP and ATP (rat receptor). Unlike the P2Y2 receptor it is not blocked by suramin. The P2Y6 receptor is distributed in the cardiovascular system and brain. It has a preference for UDP and 1,2-di-(4-isothiocyanatophenyl)ethane (MRS2567) is a selective antagonist. UDP also acts on receptors for cysteinyl leukotrienes. The P2Y11 receptor is blocked by suramin (and reactive blue 2) and it prefers ATP as an agonist. P2Y12 and P2Y1 receptors are similar in that they are both activated by ADP and more potently by 2MeSADP. P2Y12 receptor antagonists are effective in clinical use to inhibit platelet aggregation; the receptor is also involved in inhibition of neuronal cells. Antagonists include N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-βγ-dichloromethylene-ATP (AR-C6931MX or cangrelor), AZD6140, and active metabolites of clopidogrel and prasugrel. The P2Y13 receptor is found in nerve cells and immunocytes and is activated similar to the P2Y12 receptor, but is selectively blocked by 6-(2′-chloro-5-nitro-azophenyl)-pyridoxal-α5-phosphate (MRS2211). The P2Y14 receptor is activated by UDP-glucose.
Sensory enterochromaffin cells
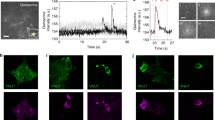
5-HT release from EC cells activates secretory, peristaltic, and vasomotor reflexes and contributes to the coordination of these reflexes. As shown in Fig. 2, EC cells with long basally located fine projections containing serotonin and sometimes connecting to neuron-like structures could be observed; other cells had short blunt processes or were dividing EC cells. A provocative possibility is the recent speculation that EC cells may even function as primary neurons [44].
Photomicrographs showing serotonin immunolabeled, formalin-fixed EC cells dispersed from the colonic mucosa. All cells have luminal projections. From the left, four serotonin-positive cells with one or two basally located, axon-like projections are seen. The fourth of these cells connects with a neuron-like structure. Right: a cell with short and blunt basal projections. The EC cells are in some cases attached to neighboring mucosa cells. (Modified from [44] by permission)
EC cell dysfunction is implicated in malignant carcinoid, dumping, and irritable bowel syndromes, as well as IBD. 5-HT secretion from carcinoid tumors is one of the putative mediators of carcinoid diarrhea that can be relieved by 5-HT antagonists. 5-HT3 antagonists or a partial 5-HT4 agonist (i.e., tegaserod) are beneficial in treating diarrhea and constipation predominant symptoms in patients, respectively [4, 67, 97]. Mucosal defects in 5-HT signaling occur in ulcerative colitis, diarrhea (or constipation) predominant IBS [24] or experimental colitis [67]. A better understanding of the signaling mechanisms, function, and dysfunction of EC cells is essential in understanding gut reflexes and symptoms of several diseases.
Despite the obvious importance of EC cells and 5-HT release in normal or disease states, our understanding of purinergic or other mechanisms involved in the regulation of 5-HT release is limited. Tissue studies are of limited use in helping us understand the complex regulation of 5-HT release because of potential interference from mediators released from neighboring cells. The human carcinoid BON cell line is a suitable EC cell model to study receptor and intracellular signaling pathways regulating 5-HT release [34].
A clone of BON/EC cells that is 99% enriched in 5-HT is a suitable EC model for population responses or for single cell functional studies. BON cells retain many of the mechanosensitive and chemosensitive properties of non-transformed cells, they respond to nutrients (glucose), mechanical activation (shaking, touch, stretch, volume changes, pressure), as well as receptor activation (purinergic, muscarinic, neurotensin, 5-HT, VIP, adrenergic, somatostatin, cholecystokinin, dopamine, bradykinin, etc.). A comparison of EC and BON cells indicates similarities in expression of receptors, ion transporters, ion channels, chemical mediators, or other mechanisms reviewed by Cooke and Christofi [25]. In addition to 5-HT, they secrete or contain adenosine, ATP neuron-specific enolase, synaptophysin, chromogranin A, neurotensin, vasoactive intestinal peptide (VIP), prostaglandins, and others. BON cell injection into nude mice can also serve as a model of carcinoid tumors [35].
Chemosensitivity of EC cells
EC cells have microvilli protruding into the lumen raising the possibility that they may “taste” the luminal contents. The nutrient monosaccharide D-glucose (10-100 mM) or the nonmetabolizable α-D-glucopyranoside (not fructose) caused a graded increase in 5-HT release in BON cells that was not caused by changes in osmolarity. Phloridzin blocked the effect of D-glucose by blocking D-glucose uptake [59, 87]. The role of purinergic modulation of nutrient activation of 5-HT release remains unknown.
Mechanosensitivity of EC cells
Mechanosensitivity in EC cells is poorly understood and the mechanosensitive elements remain unknown. Brush stroking, compression of the mucosa or villi, rotational stroking, or pressure/volume changes used to evoke mucosal secretomotor reflexes in the gut are not suitable to elicit 5-HT release in isolated EC/BON cells. Mechanical forces in the gut are complex and likely represent a composite of forces (pressure, shear force, centrifugal force, stretch, deformation, compression, touch, etc.). We succeeded in employing three different mechanical stimuli to elicit responses in EC cells to study purinergic regulation of mechanosensitive 5-HT release. Rotational shaking of cultured cells simulates forces that are generated in the intestinal lumen (i.e., shear forces, centrifugal forces, changes in hydrostatic pressure) and releases 5-HT. Pressures of 75 mmHg or greater (normally associated with painful stimulation) applied to cells in a pressure chamber or laminar shear stress of 1–2 dyne/cm2 applied in a parallel plate flow chamber evoked 5-HT release [26].
Second messengers in mechanosensitivity
Balloon distension and puffs of nitrogen gas/pressure stimulate 5-HT release and trigger a reflex secretory response via submucous neurons in intact mucosa/submucosa preparations [84]. Rotational shaking of tissue preparations or the EC/BON cells in culture predominantly activates a Gαq/PLC/inositol triphosphate (IP3)–Ca2+ signaling pathway leading to 5-HT release [59]. A separate Gs/Gi/AC/PKA (protein kinase A)/cAMP signaling pathway provides a minor contribution to mechanosensitive 5-HT release [18].
Dual modulation of mechanosensitivity by P2Y1 and P2Y12 receptors
Recent evidence from pharmacological and molecular studies support the hypothesis that dual modulation of 5-HT release occurs via excitatory P2Y1 receptors coupled to the Gαq/PLC/IP3-Ca2+ signaling pathway leading to 5-HT release [114] and inhibitory P2Y12 receptors coupled to AC (Christofi, Wunderlich, Xue, Cooke, unpublished observations; [120]). Touch/stretch evokes a Ca2+ response in BON/EC cells that is used to study purinergic regulation of mechanosensitivity. In preliminary studies, touch Ca2+ responses in EC cells were inhibited by apyrase (ectonucleotidase) whereas a 5′-ectonucleotidase inhibitor ARL 67156 augmented responses indicating that a nucleotide is involved in mechanosensitivity. When single cells are examined and analyzed according to inhibition or augmentation of the Ca2+ response, dual modulation of the response is revealed in subsets of EC cells. Therefore, the P2Y1 receptor antagonist MRS2179 could either abolish the touch Ca2+ response or augment the response in different subsets of EC cells. MRS2179 could block touch-evoked Ca2+ responses in cells responsive to the P2Y1 receptor agonist 2MeSADP. The Ca2+ response evoked by 2MeSADP was either inhibited or augmented by MRS2179 in subsets of EC cells; an additional component of the touch Ca2+ response was blocked by the P2 receptor antagonist PPADS [114].
In molecular signaling studies, when the human P2Y1 receptor was overexpressed in BON cells with naitive P2Y receptors, it caused additional mobilization of Ca2+ in the cells and augmented 5-HT release. A P2Y1 receptor silencing RNA reduced receptor expression leading to a reduction in 5-HT release. In heterologous 1321N1 cells, transfection of human P2Y1 receptors enabled the cells to generate a Ca2+ response to mechanical stimulation. P2Y1 receptor antagonists MRS2179 or A3P5P abolished the Ca2+ response in 1321N1 cells or 5-HT response in BON cells that overexpressed the human P2Y1 receptor [120]. Overexpression of the P2Y12 receptor in BON cells and/or HEK293 cells nearly abolished mechanically evoked 5-HT release (BON) and cAMP (both cell types). Blockade of P2Y12 receptors by 2-MeSAMP augmented the cAMP responses. 5-HT release was augmented after knockdown of P2Y12 receptors in BON cells; silencing of P2Y13 receptors had no effect. Therefore, molecular studies are consistent with pharmacological studies and suggest that dual purinergic modulation of mechanosensory signaling via P2Y1 and P2Y12 receptors (that recognize both MRS2179 and 2MeSADP) leads to 5-HT release in EC cells. The P2Y1 and P2Y12 receptors may provide new targets for modulation of mucosal secretory reflexes and are potential therapeutic targets, as shown for platelets [1]. The role of other receptors expressed in EC/BON remains unknown. Messenger RNA transcripts for P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, and P2Y13 exist in BON cells [25, 26].
Adenosinergic modulation of 5-HT release
Endogenous adenosine modulates the basal release of 5-HT from EC/BON cells. Adenosine deaminase caused a dose-dependent increase in 5-HT release (Fig. 3a). The nucleoside uptake inhibitor S-(p-nitrobenzyl)-6-thioinosine (NBTI) prevented mechanically evoked 5-HT release (Fig. 3b) by elevating endogenous adenosine (Fig. 3c). Overall, endogenous adenosine is sufficient to provide ongoing inhibition of basal and mechanically evoked 5-HT release. The adenosine A3 receptor is activated by endogenous adenosine since baseline 5-HT release was augmented by an A3 receptor antagonist MRS1191 and this was sensitive to an A3 receptor agonist IB-MECA (Fig. 3d); the A3 effect was restricted to the Ca2+ signaling pathway. The A1 receptor also contributes, and an A1 receptor antagonist (CPT), an A3 receptor antagonist (MRS1191), or adenosine deaminase could also augment the touch/stretch Ca2+ response (Fig. 3e). Touch Ca2+ responses occur via a Gαq/PLC/IP3 pathway that is the predominant mechanism in 5-HT release from EC cells. 5′-N-methylcarboxy aminoadenosine (MECA) elevated both 5-HT and cAMP levels in BON cells. A2 receptor antagonists could suppress mechanically evoked 5-HT release indicating the endogenous adenosine can contribute to stimulation of 5-HT release via A2 receptors. Together, these and other findings indicate that endogenous adenosine can activate inhibitory A3/A1 receptor–Gαq/PLC/IP3–Ca2+ and excitatory A2a/A2b receptor–AC/cAMP signaling pathways on EC cells to regulate 5-HT release; A1, A2a, A2b, and A3 receptors or mRNA transcripts are present in both BON/EC cells, gastric and intestinal 5-HT-ir carcinoid tumors [18]. Recent isolation of ileal EC cells [58] may permit further study and comparison of purinergic signaling mechanisms in normal and diseased human ileum. Real-time electrochemical detection of local mucosal 5-HT release is also very promising [5].
Regulation of 5-hydroxytryptamine (5-HT) release by endogenous adenosine release from BON cells via inhibitory A1/A3 receptors and excitatory A2 receptors. a The enzyme ADA caused a dose-dependent increase in 5-HT release, indicating that release of endogenous adenosine provides an ongoing inhibition of 5-HT release. b In the same cells as a, NBTI blocked adenosine uptake and abolished mechanically evoked 5-HT release. c Adenosine release during resting/unstimulated conditions (Base) and mechanical stimulation (MS) is augmented in the presence of the adenosine uptake inhibitor S-(p-nitrobenzyl)-6-thio-inosine (1 μM NBTI). d Chemical stimulation with IB-MECA decreased basal 5-HT and the A3R agonist cAMP levels. The A3R antagonist MRS1191 (10 nM) augmented 5-HT release but not cAMP levels (p < 0.05). The MRS1191-evoked 5-HT response was sensitive to IB-MECA inhibition. e Adenosine A1 and A3R inhibitory components of touch-evoked Ca2+ responses in enterochromaffin/BON cells. The A3R antagonist MRS1191 augmented the touch Ca2+ transient. Chronic exposure to the A1 antagonist CPT for 24 h caused a significant augmentation in the touch Ca2+ response. The stimulatory effects of CPT and MRS1191 were additive on the touch Ca2+ response. Adenosine deaminase (5 units/ml)-induced augmentation is reduced by the A3R agonist IB-MECA (50 nM). f A2A/A2B antagonists CGS and AL (alloxazine) inhibited mechanically evoked 5-HT release. Baseline values (pmol/well per 30 min) of 5-HT and/or cAMP are at the top of figures with the number of experiments indicated in parentheses. IB IB-MECA, MRS MRS1191, ME MECA, AL alloxazine, CGS CGS 21680, MS mechanical stimulation by rotational shaking at 80 rpm, NS not significant. (Modified from [59] by permission)
Intestinal secretory reflexes
Hubel’s [51] pioneering spirit and studies demonstrated that electrical field stimulation of tissue setup in Ussing chambers can stimulate transepithelial short-circuit current (Isc) changes indicative of ion transport. His studies provided proof that submucous neurons could regulate chloride secretion. This sparked the beginning of a new era of investigation on the influence of the ENS on epithelial secretion and secretomotor reflexes.
The secretory reflex can be activated by nutrients or mechanical stimulation or exposing the gut to food antigens regarded as foreign or to enteropathogenic microorganisms. The mucosal surveillance and immune defense system is exposed to an estimated 1012 different antigens per day that mobilizes both intrinsic and extrinsic nervous systems, hormones, paracrine substances, and immune mediators to modulate chloride secretory rates.
The net flux of ions into the gut lumen is the balance of secretion and absorption. Basal secretion or “secretory tone” is also the net flux of ions and fluid into the intestinal lumen that is dependent on the spontaneous release of endogenous paracrine, autocrine, or neurocrine mediators. Secretory tone is assessed from changes in basal Isc, a measure of chloride secretion. The basal Isc is under ongoing neural stimulation. Data with P2 receptor antagonists (PPADS, suramin) or P1 receptor antagonists (CPT), nucleotidases, or nucleotidase inhibitors indicated that endogenous nucleotides stimulate, while endogenous adenosine inhibits basal Isc [29]. However, basal secretion is not affected by P2X or P2Y1 receptors because neither of NF279 or MRS2179 influenced Isc. Overall, adenosine, nucleotides, somatostatin, and prostaglandins all contribute to “secretory tone” [26, 27].
P2Y1 receptors and mucosal stroking-evoked neurosecretory reflexes in the colon
Mechanical or chemical activation of the mucosa can lead to an intestinal neural reflex and an increase in ion transport and fluid secretion. Very few studies have investigated purinergic regulation of secretomotor function [18, 27–29]. Mucosal distortion by brush stroking or distension can elicit the reflex (Fig. 4).
Modified Ussing chambers used for mucosal stroking studies to evoke a reflex neurosecretory response. a Vertical configuration of the chamber permits a 2-mm brush to be placed on the mucosal surface. A single stroke represents the directional movement of the brush from one side to the other on the surface of the mucosa. b A complete 360° turn of the brush is another variation for stimulating reflexes; stroke (1 s) is reproducible every 5 min for a 40-min period. c Distention is accomplished by removing small volumes of fluid from the serosal side. Drugs are perfused separately on the serosal or mucosal surfaces of the mucosa-submucosa or whole thickness colonic or small bowel tissues. The Isc/short-circuit current nulled out the spontaneous potential difference (PD) and was used as a measure of chloride secretion in the distal colon. (Modified from [25] by permission)
Brush stroking of the mucosa causes an intestinal neural reflex response and an increase in Isc indicative of electrogenic chloride ion transport. The brush stroking reflex in the rat colon mucosa-submucosa is reduced by MRS2179 or apyrase and further inhibited by tetrodotoxin (TTX). 2MeSADP, a mixed P2Y2/P2Y4 agonist UTP, or ATP (P2 receptor agonist) evoked neural and non-neural secretory responses. Mucosal touch/distension evoked fluo-4/Ca2+ responses in submucous neurons were also inhibited by apyrase or blocked completely by MRS2179 (Fig. 5); MRS2179 only reduced Isc in stroking reflexes. The potency profile of nucleotides for reducing Isc is different from that for touch Ca2+ responses [18]. P2Y1 immunoreactivity was identified in a majority of VIP, NOS, calretinin, NPY, or somatostatin neurons but not SP or calbindin submucous neurons. P2Y4 immunoreactivity occurred mainly in the cell somas of NPY neurons (93%). P2Y2 immunoreactivity occurred in a minority of SP, VIP, NPY CGRP varicose fibers (5-20%) and those surrounding calbindin neurons [18, 26]. Purinergic responses could be distinguished based on stroking reflexes, touch-evoked Ca2+ reflexes, actions of nucleotides, or sensitivity to antagonists. These and other data suggest that several nucleotides may contribute to mechanically evoked secretomotor reflexes. Nucleotides differentially activate P2Y1, P2Y2, and P2Y4 receptors located at putative pre- or postsynaptic sites on submucous neurons. In rat hippocampus, ATP may activate presynaptic P2Y1, P2Y2, and P2Y4 receptors to inhibit glutamate release [91]; presynaptic P2Y1 and P2Y2 receptors also occur in the rat submucous plexus. A working model of the purinergic neural circuitry in the rat colon is shown in Fig. 6.
Laser confocal imaging of touch-induced purinergic Ca2+ signals in neurons of the intact microdissected submucous plexus of the rat distal colon. a Perfusion chamber used to record touch-induced Ca2+ responses in the neurons. It includes a recording chamber made of a 35-mm microwell dish with a 1-cm, number 0.0 cover glass in the center of the bottom of the dish for visualizing the fluorescent neurons. A portion of the dish is embedded with a 2-mm thick layer of Sylgard shaped around a half moon at one end of the dish. Perfusion with oxygenated buffer or drugs is exactly opposite to the suction. The microdissected M-SMP preparation is stretched and fixed with many (15-20) micropins along the Sylgard layer. The remaining SMP without mucosa is stretched over the glass cover slip of the dish and secured with magnetic metal feet (not shown). A large ganglion of the intermediate layer of the submucous plexus is viewed with a 40× oil immersion objective (N.A. 1.3). b Inset shows Fluo-4/AM loaded neurons representing second order neurons that are visualized. c Side view of the tissue: The submucous plexus layer is located flat on the bottom of the dish and the mucosa/submucosa lifts over the Sylgard ends at each side of the half moon. The tissue is touched for 20 s on the mucosal surface of M-SMP portion that is stretched across the Sylgard with a fire-polished glass pipette to evoke a Ca2+ response in the neurons—the tissue is free to move/stretch downward during the touch, since it is located a distance of 2 mm above the glass bottom. d–g The P2Y1 antagonist MRS2179 or TTX abolish the touch-induced Ca2+ fluorescence response. A representative example of the effects of MRS2179 and TTX on touch-induced Ca2+ transients in a single ganglion (seven neurons in one ganglion). A 20-min exposure to the selective P2Y1 antagonist MRS2179 (10 μM) abolished the touch response in the neurons. In the presence of 0.2 μM TTX, the touch response decreased to below baseline levels. Arrows indicate touch-responsive cells. h Pooled data for the peak effects of MRS2179 and TTX; averaged transients from 30 neurons from 3 to 4 separate ganglia. i Breaking down endogenous nucleotides reduces the touch-induced Ca2+ response in the neurons of the intact M-SMP. The touch response was determined in the presence of 5 units/ml apyrase exposed for 10 min. Apyrase reduced by 50% the control touch response (n = 4 neurons, p < 0.01). Values are means ± SEM. (Modified from [18] by permission)
Mechanical activation of purinergic neural circuitry in rat distal colon. Mucosal brush stroking or touch/stretch releases nucleotides that may include ATP, UTP, and ADP that activate neural P2Y1, P2Y2, and P2Y4Rs at pre- or postsynaptic sites of submucous neurons leading to a net increase in Isc/chloride secretion. Intrinsic primary afferent neurons do not express P2YRs on their cell somas, but receive strong synaptic inputs from extrinsic fibers (i.e., pelvic origin) with P2Y2Rs forming baskets of varicose fibers surrounding their cell somas. A very tiny subset of VIP secretomotor neurons expresses P2Y2R. P2Y2Rs, P2Y1Rs, and P2Y4Rs are coexpressed on > 90% of NPY/VIP putative secretomotor neurons that also express SP Rs. P2Y1Rs are also expressed on putative somatostatin (SOM) neurons. Activation of P2Y1Rs leads to a rise in intracellular free Ca2+ levels in the secretomotor neurons by ATP, UTP, or ADP. A nicotinic cholinergic synapse has not been ruled out. NOS neurons and CALR/CHAT cholinergic neurons also express P2Y1Rs, but the functional role of these subsets of neurons is unknown. P2Y2Rs are discretely localized on some extrinsic fibers of SP, VIP, NPY, CGRP, or cholinergic origin presumed to be involved in synaptic modulation of transmitter release. (Modified from [18] by permission)
ARL 67156 augmented, whereas apyrase, atropine, or TTX could inhibit stroking-evoked reflex Isc responses in guinea pig distal colon (Fig. 7a). The agonist potency profile of 2MeSADP > UTP ≥ UDP for stimulation of neural secretion supports a P2Y1 receptor (Fig. 7b), but does not exclude others. MRS2179 reduced the stroking response by 54% and the effect of 2MeSADP by 70%. P2 receptor antagonists PPADS and suramin had additive inhibitory effects. The P2X1/3 receptor agonist αβMeATP caused stimulation of Isc, and a desensitizing concentration reduced stroking-evoked secretion but did not affect the 2MeSADP response. Cell bodies of guinea pig colonic submucous neurons expressed P2Y immunoreactivity. About 20-50% of submucous neurons expressed P2Y1 and P2Y2 receptors. P2Y immunoreactivity (P2Y1, P2Y2, or P2Y4) was virtually undetectable in any varicose fibers. P2Y1 immunoreactivity was abundant in putative cholinergic secretomotor neurons and fewer than 2% of NPY/cholinergic secretomotor neurons [71]. In contrast, P2Y2 immunoreactivity was found in all NPY secretomotor neurons and 30% of calbindin/intrinsic sensory neurons. P2Y4 is of minor significance in submucous neurons. In guinea pig, mucosal stroking may activate putative P2X1/3, P2Y1, and P2Y2 receptors located at postsynaptic membranes of submucous neurons leading to chloride secretion; P2Y1 receptors on EC also contribute. Based on the available evidence, it was hypothesized that a separate P2Y2 neural circuit pathway exists in guinea pig that is not activated by stroking the mucosa [25, 27]. In both rat and guinea pig, receptors or mRNA transcripts exist for P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptors in the submucosa, and therefore influence of other receptors in stroking reflexes deserves further attention. Species differences exist between mucosal stroking reflexes in rat [18] and guinea pig [27] distal colon in purinergic regulation, and no information exists for the human GI tract.
Mucosal stroking releases endogenous ATP/nucleotides that activate excitatory neural P2Y1Rs leading to an increase in Isc/chloride secretion in the guinea pig colon. a The ATPase/5′-nucleotidase inhibitor 6-N,N-diethyl-β,γ-dibromomethylene-D-adenosine-5′-triphosphate trisodium (10 μM, ARL 67156 or ARL) prevents hydrolysis of ATP and enhanced reflex-evoked Isc (n = 6, *p < 0.05), whereas 10 U/ml apyrase hydrolyzes ATP and inhibited reflex-evoked Isc responses (n = 4). This indicates that endogenous nucleotide release contributes to the stroking response. b Concentration-response curves for nucleotides in the presence of 1 μM CPT (n = 4-23), an A1 receptor antagonist used to block eADO effects. The rank order of potencies of nucleotide agonists for stimulation of Isc was 2MeSADP = 2MeSATP > UTP = UDP that indicates a P2Y1R interaction. c The selective P2Y1R antagonist MRS2179 suppresses ~50% of the stroking response. d The Isc response to the P2Y1R agonist 2MeSADP was nearly abolished by the P2Y1R antagonist MRS2179 (10 μM, n = 4, p < 0.05). All experiments were done in the presence of 1 μM CPT to eliminate inhibitory influence of A1 receptor activation by endogenous adenosine (n >/ 4, *p < 0.05) (Modified from [27] by permission)
Neurogenic mucosal secretion and P2Y1 receptors in small intestine
In the guinea pig small intestine, it was shown that neurogenic mucosal secretion is mediated by the P2Y1 receptor that may be expressed on VIP secretomotor neurons. MRS2179 blocked the neurally mediated ATP-evoked response with an IC50 of 0.9 μM. TTX could suppress or abolish the ATP response. The P2Y1 receptor was coupled to PLC/IP3/Ca2+–calmodulin/protein kinase C signaling pathway [37] like that on EC/BON cells.
Purinergic modulation of local inhibitory reflexes
Local inhibitory reflexes to the circular muscle evoked by mucosal application of nutrient amino acids in guinea pig jejunum are partially blocked by antagonists to P2X receptors suggesting involvement of ATP [46]. The receptor subtype(s) have not been characterized. Effects of nutrients on local secretomotor reflexes and involvement of purinergic signaling in the coordination of motility and secretion remain unclear, although some data are emerging on adenosine A3 and A1 inhibitory receptors (see later section on “Neurogenic diarrhea”; [8, 9]).
Fluo-4/Ca2+ imaging studies are providing new insights into the functional purine receptors involved in the excitability and synaptic transmission in intact neural circuits of the submucous plexus of the human gut. A recent study indicated that the submucous plexus from Roux-en-Y patients is a suitable model to study synaptic transmission in the human enteric nervous system [115]. It was shown that the P2Y1 Gαq-PLC/IP3 Ca2+ signaling pathway, N-Ca2+ channels, nicotinic receptors, and extrinsic nerves are involved in neurotransmission. Pharmacological findings suggest that inhibitory adenosine A3 receptors modulate nucleotide and cholinergic transmission in the human enteric nervous system.
In the guinea pig small intestine, electrophysiological studies revealed that AMP activates presynaptic inhibitory A1 receptors to suppress neurotransmission and postsynaptic A2a receptors to elicit a slow excitatory postsynaptic potential (EPSP)-like response. Signaling pathways coupled to A2a receptors include AC, PLC, PKC, and calmodulin-dependent kinases [41]; whether AMP can also activate A2b or A3 receptors remains unclear.
Endogenous adenosine and A1 receptors in mucosal reflexes
The role of endogenous adenosine in the physiological regulation of mucosal secretory reflexes was investigated in pharmacological studies [29]. At nanomolar concentrations, the A1 antagonist 8-chlorophenyltheophylline (CPT) caused a concentration-dependent enhancement of the stroking reflex. The effect still occurred in the presence of an A2a receptor antagonist 8-(3-chlorostyrl) caffeine. Adenosine deaminase (5 U/ml) also enhanced the reflex secretory response, whereas the nucleoside uptake blocker NBTI inhibited it. The A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA) inhibited reflex-evoked Isc (Fig. 8a; EC50 = 6 nM) and its effect was also blocked by CPT. In experiments with either piroxicam to block the prostaglandin (PG)-mediated pathway or N-acetyl-5-hydroxytryptophyl-5-hydroxytryptophan amide to block the 5-HT mediated pathway, CCPA reduced or abolished the residual reflex Isc response (Fig. 8b). CCPA could abolish the reflex response to a pulse of 5-HT (Fig. 8c) without affecting TTX-insensitive epithelial responses to carbachol or forskolin. A1 immunoreactivity was expressed in submucous neurons, in presynaptic varicose nerve endings (synaptophysin-positive neurons), and in glia. The chemical coding of those submucous neurons is not known. Overall, available data suggest that endogenous adenosine provides a physiological brake to suppress reflex-evoked Cl- secretion elicited by stroking the mucosa by acting at A1 receptors located on EC cells [17, 18] and in the ENS [29]. Adenosine acts at A1 receptors to suppress both the PG and 5-HT sensory limbs of mucosal secretory reflexes [29]. Our understanding of the role of A1 receptors in the modulation of mucosal reflexes is restricted to the guinea pig colon; however, it is likely this effect will occur in other regions, given that the A1 receptor provides inhibition of synaptic transmission in myenteric and submucous neurons, and in other regions including stomach antrum, jejunum, and ileum [8, 20, 22].
Effect of CCPA on stroking-evoked change in Isc. A Concentration-response curve (EC50 = 6 nM, n = 3-6). B Effect of CCPA (0.1 μM) on 5-HT- and PG- mediated limb of reflex pathway. a Presence of 5-HT limb after blockade of prostaglandin synthesis with 10 μM piroxicam (PIR) (n = 5). b Presence of PG limb after blockade of 5-HT limb with 1–10 μM N-acetyl-5-hydroxytryptophyl-5-hydroxytryptophan amide (HTP) (n = 6 or 7). Con control, p < 0.05 from control and PIR or HTP. C Effect of CCPA (0.1 μM) on change in Isc evoked by a mucosal pulse of 5-HT (1 μM) (n = 3); p < 0.05 (Modified from [29] by permission)
Distribution of adenosine, P2X, and P2Y receptors and gene products in ENS
Immunohistochemical, gene chip microarray, quantitative reverse transcriptase polymerase chain reaction (RT-PCR), or in situ hybridization data have identified various purinergic receptors in the gut of various species. These include A1, A2a, A2b, A3, P2X2, P2X3, P2X5, P2X7, P2Y2, P2Y4, P2Y6, P2Y12, and P2Y14 receptors in the ENS of guinea pig, mouse, rat, or human intestine [14, 17–19, 25, 27, 29, 49, 93, 94, 108, 109, 117–119].
Differential gene and receptor expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system
Adenosine A1, A2a, A2b, and A3 receptor gene products were differentially expressed in neural and non-neural layers of the human jejunum, ileum, cecum, and colon as well as T-84, HT-29, T98G, and BON/EC cells or in PGP 9.5-ir enteric neurons [19]. Table 1 summarizes adenosine receptor mRNA expression levels in the different layers and cells using RT-PCR. With the exception of the A1 receptor, mRNA transcripts for A2b, A2a, and A3 receptors are expressed throughout the GI tract in epithelial cells, mucosa, and submucosa suggesting a significant role in secretomotor behavior of the human gut.
Cellular localization of adenosine receptor immunoreactivity is summarized in Table 2. Adenosine A3 immunoreactivity is also expressed in a majority of substance P (~60%) and < 10% of VIP submucous neurons. In contrast to A3R receptors, A2b immunoreactivity is prominent in 50% of VIP neurons and not expressed in SP neurons in jejunum, but is expressed mainly in non-VIP neurons in colonic submucous neurons. Therefore, A2b receptors and A3 receptors may serve different roles in secretomotor function in the two regions by acting at putative secretomotor or sensory neurons, respectively.
A2a immunoreactivity is found in a subset of VIP neurons. Varicose fibers in submucous ganglia label for A2a or A2b immunoreactivity. A2a and A2b immunoreactivity is prominent in submucous neurons, but only expressed in a minority of myenteric neurons. Immunoreactivities for A2a and A2b receptors are colocalized in submucous neurons of the jejunum and colon (and jejunal myenteric neurons) as well as in epithelial cells of the jejunum (only region studied). A2b immunoreactivity was also intensely expressed in s-100 positive glia in both nerve plexuses.
A1 immunoreactivity is abundantly expressed in submucous neurons of the colon, but was undetectable in submucous neurons of the jejunum. This is consistent with functional studies on synaptic Ca2+ responses in submucous neurons illustrating a lack of effect of A1 receptor antagonists expected to block ongoing inhibitory effects of endogenous adenosine at neural receptors and enhance synaptic transmission, as revealed in other tissues with functional A1 receptors (see later discussion; [9, 21]). In addition, strong A1 immunoreactivity is found in epithelial cells of the human colonic mucosal glands, and mRNA and proteins expressed in two human epithelial cell lines. Therefore, A1 receptors could potentially play a role in submucosal neural reflexes, leading to suppression of intestinal secretion in the human gut as reported for the guinea pig colon [29]. The diverse but receptor-specific localization and expression of the four adenosine receptors in submucous neurons, glial cells, or epithelial cells, and the colocalization of A2a and A2b receptors in submucous neurons or glandular epithelia, suggests a prominent and complex role for adenosine in the modulation of enteric neural reflexes that control secretomotor functions in the human digestive tract. Functional studies on adenosine receptors in human gut would be of physiological and clinical importance.
Distribution of P2X and P2Y receptors in the enteric nervous system
P2X2 immunoreactivity has been identified in guinea pig ENS [14, 50, 111]. In submucous ganglia of the guinea pig ileum, P2X2 immunoreactivity occurred in all VIP-positive neurons and calbindin-positive neurons making up about 50% of the neurons. In contrast, there was not any P2X2 immunoreactivity in NPY or calretinin-positive neurons. Overall, P2X2R are expressed in both nerve plexuses, in inhibitory motor neurons, IPANs of the myenteric plexus in stomach and intestine, on gastric endings of vagal afferent fibers, VIP noncholinergic secretomotor neurons, and IPANs of the submucous plexus [14]. In contrast to P2X2, P2X3 immunoreactivity in the submucous plexus occurs in most calretinin neurons that represent cholinergic neurons projecting to mucosa or arterioles, and make up ~12% of the population [10]; therefore, the activity of some secretomotor neurons is likely to be modulated by P2X3 receptors in guinea pig.
P2X2 and P2X3 immunoreactivity was also identified in nerve fibers in enteric ganglia, interganglionic fiber tracts, in the muscularis as well as in the perivascular plexus of the guinea pig gallbladder. P2X2 or P2X3 neurons displayed immunoreactivity for VIP (> 90%) and NOS (~50-60%) [93]. The function of these receptors remains unknown.
P2X2 and P2X3 immunoreactivity was also localized in rat ENS, by in situ hybridization or mRNA levels [119]. Receptors were identified throughout the GI tract in both myenteric and submucous neurons. P2X2 immunoreactivity (56% ileum, 45% distal colon) and P2X3 immunoreactivity (62%, 40%) was abundant in submucous neurons. P2X2 immunoreactivity in submucous plexus colabeled for calbindin or calretinin in 30-50% of neurons. P2X3 immunoreactivity neurons expressed calretinin (40%) and calbindin (30-75%). Coexpression of P2X3 immunoreactivity in calbindin neurons also occurred in colorectum [116] but not in those in rat ileum [85, 108]. At least in the mouse, AH sensory (not S/type 1) neurons did express P2X3 immunoreactivity [7]. Species and region differences may exist in distribution of the P2X2 and P2X3 receptors in submucous neurons (see [7, 14, 49, 93, 111, 118]). The P2X1, P2X4, or P2X6 receptor was undetectable in mouse ENS. The P2X5 receptor is widely distributed in mouse GI tract in enteric ganglia in both nerve plexuses. In the submucous plexus, P2X5 immunoreactivity discretely colocalize with 22% of calretinin, 9% of calbindin, 6% of nitric oxide synthase, and 68% of VIP-positive neurons. Therefore, the P2X5 receptor may be distributed in secretomotor and intrinsic sensory neurons. The mouse P2X5 receptor could form heteromultimers with a unique pharmacology [94].
P2X3 immunoreactivity has also been identified in submucous (and myenteric) neurons as well as in some axons/dendrites of the human colon [36, 122], but the identity of the subtypes of human neurons expressing P2X3 immunoreactivity remains unclear. Future studies should include detailed analysis of the distribution of purinergic receptors in the human ENS.
P2Y2 immunoreactivity occurs in neurons and fibers in both myenteric and submucous plexuses in the corpus of the stomach, jejunum, ileum, and colon of the guinea pig [118]. P2Y2 immunoreactivity is prominent in neurons with Dogiel type I morphology. All P2X3 neurons and none of the P2X2 neurons in the submucous plexus (or ~50% in myenteric neurons) coexpressed P2Y2 immunoreactivity throughout the GI tract. A majority of calretinin and NPY submucous neurons also expressed P2Y2 immunoreactivity. Submucosal neurons with P2X3 and P2Y2 receptor immunoreactivity with Dogiel type I morphology may represent a subset of S/type 1 neurons with Dogiel type I morphology that display both a fast P2X and slow P2Y membrane depolarization [3]. It has been suggested that a P2X2 or P2X7 receptors may mediate the fast P2X response in neurons that only display a fast response [3, 14, 49, 119]. Lack of P2Y2 immunoreactivity in calbindin submucosal neurons of the guinea pig gut argues against their expression on IPANs [39]. Regional differences occur in the colocalization of P2Y2 immunoreactivity with P2X3 immunoreactivity or in other types of neuronal markers. These receptors may play a role in regulation of mucosal secretory glands and the local vasculature.
The P2Y6 receptor is widely distributed in myenteric but not submucous ganglia of the stomach, jejunum, ileum, and colon (in guinea pig), whereas the P2Y12 receptor is also widely distributed in submucous ganglia. P2Y12 and P2Y6 receptors and neurons likely play different roles in the gut. A distinction between their localization is that P2Y12 immunoreactivity (not P2Y6) is also expressed in calbindin intrinsic sensory neurons and not calretinin or NOS (in both plexuses). P2Y6 immunoreactivity is instead found in NOS and calretinin-positive neurons. Therefore, the P2Y12 receptor may play a role in sensory signaling in mucosal secretomotor reflexes.
Mucosal epithelial cell lines and ion transport
In human epithelial carcinoma cell lines (HCT8 and Caco-2), cells express mRNA for P2X1, P2X3, P2X4, P2X5, P2X6, and P2X7 and P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12 receptors. In addition, P2Y1, P2Y2, and P2X1-7 receptor proteins are expressed in these cells. Functional studies on cell proliferation and apoptosis using selective agonists and antagonists suggested involvement of P2X7, P2Y1, P2Y2, and P2Y4 receptors but their role in secretion awaits further study [31].
ENaC and CFTR channels
ENaC mediate electrogenic absorption of Na+ in intestine (and other epithelial tissues). ENaC in colonic epithelia is expressed together with the CFTR Cl- channels. Stimulation of purinergic receptors by ATP or activation of CFTR stimulate chloride secretion and inhibit amiloride-sensitive Na+ transport (ENaC). Inhibition of ENaC by nucleotides is not via CFTR-mediated ATP release [62]. ATP or UTP acting at P2YRs also activate Ca2+-dependent Cl- channels. Na+ absorption in mouse colon (and airway epithelia) is inhibited by cell shrinkage (change in volume) by a mechanism that does not interfere with purinergic (or CFTR) mediated inhibition of ENaC [101]. A recent study in P2Y2 and P2Y4 knockout mice suggests that only the stimulation of the luminal P2Y2 receptor mediates inhibition of electrogenic Na+ absorption via epithelial Na+ channels (ENaC) in mouse colon. In contrast, luminal P2Y2 and P2Y4 receptors stimulate K+ secretion [74].
Luminal P2 and ion transport
P2 receptors are found in both the basolateral and luminal membranes. A recent review by Leipziger (2003) summarizes the different P2X and P2Y receptors, their functions, and intracellular signaling pathways in all epithelial tissues.
Relevant information on gut luminal P2 receptors is summarized in Table 3. Several generalizations can be made from Table 3. Epithelial cells respond well to ATP and UTP. P2Y2 and P2Y4 receptors are very prevalent luminal receptors involved in Cl-, HCO3 -, K+ secretion, or mucin secretion. Nucleotide stimulation of K+ secretion occurs in the distal colon, K+ secretion and HCO3 - secretion in the gallbladder (Table 3; [17, 18, 75, 90]); HCO3 - secretion also occurs in ducts and is involved in the formation of pancreatic juice. Stimulation of Cl- secretion occurs in the gallbladder, small intestine, and duct cells. Ca2+ is the main second messenger coupled to P2Y receptors, although cAMP can be involved as well. Region and species differences occur especially in P2X7 and P2Y6 receptors. Functional discrimination between P2Y2 and P2Y4 receptors is difficult because of the similar agonist potency profiles [102]. The persistence of a Cl- secretory response to UTP or ATP in P2Y2 -/- mice suggested that the response in mice could be mediated by P2Y4 receptors [32]. Indeed, loss of nucleotide regulation of epithelial chloride transport occurs in the jejunum of P2Y4-null mice [90]. P2Y4 mRNA are expressed in rat and guinea pig submucosa [17, 18, 27], murine colonic crypts [75], stomach and intestine [90, 105], and more recently in enteric glial cells [108, 109]. In the guinea pig small intestine, P2Y4 immunoreactivity is expressed in enteric glial cells (EGCs) in various regions of the GI tract except esophagus. EGCs are important regulators of barrier function, mucosal permeability, neuronal activity, and epithelial cell growth and are active participants in intestinal inflammation [13, 95]. Some discrepancy exists between studies on whether P2Y4 immunoreactivity occurs in epithelial cells, but EGCs may play a role in the modulation of ion transport in gut epithelial cells (for review see [95]).
Little evidence exists that luminal P2 receptors activate absorption, other than the preposition that a P2X7 receptor is involved in stimulation of Na+/H+ exchanger type 3-mediated Na+ absorption in rat submandibular gland ducts [66]. In contrast, data suggest that luminal fluid secretion and a diarrhea response would occur with activation of luminal P2Y receptors in small intestine (Cl- secretion) or colon (inhibition of Na+ absorption via ENaCs and K+ secretion); a diarrhea response may play a role in host defense reactions against potential pathogenic invading organisms. Another possibility is that the P2X7 receptor in villus tip cells that undergo programmed cell death and exfoliation may act as a “death signal” to insure villus cell regeneration [43]. The P2Y6 receptor is a potential therapeutic target in the treatment of cystic fibrosis gallbladder disease because UDP retained its ability to promote/stimulate Isc/ion transport changes in cystic fibrosis gallbladder epithelial cells from CFTR-deficient mice [65].
Basolateral P2Y6 stimulates NaCl secretion
Recent evidence suggests that basolateral P2Y6 receptors that are sensitive to UDP in colonic epithelia cells stimulate sustained NaCl secretion by evoking a synergistic increase in intracellular free Ca2+ and cAMP. In Xenopus oocytes coexpressing P2Y6 with the cystic fibrosis transmembrane conductance regulator (CFTR), UDP transiently activated the Ca2+-activated Cl- current and subsequently CFTR.
A2b receptors in apical secretion
Adenosine is generated at sites of tissue injury or stress including ischemia, inflammation, and tissue remodeling by ATP catabolism. Adenosine at inflammatory sites can have either proinflammatory or anti-inflammatory effects depending on the receptor or tissue. In crypt abscesses during periods of active inflammation, ectonucleotidases convert ATP derived from neutrophils to adenosine. Adenosine causes vectorial chloride and interleukin-6 secretion [33, 73, 103]. In human jejunum, an A2 receptor agonist 5′-N-methylcarboxamidoadenosine stimulates chloride secretion. A2b immunoreactivity is prominent in human mucosa or epithelial cells of the cecum, colon, and jejunum (Christofi et al. 1999; [89, 104]).
At rest, in epithelial cells, the A2b receptor is intracellular and inactive, and agonist stimulation on the apical or basolateral side of human epithelial cells induces polarized trafficking and surface expression of the A2b receptor in intestinal epithelial cells [33]. N-ethylmaleimide attachment receptor (SNARE) proteins participate in recruitment of A2b receptors that may be necessary for its signaling. The A2b receptor signals through the cAMP/protein kinase A/cAMP response-element binding protein to cause apical chloride and interleukin-6 secretion. It has been suggested that interleukin-6 would promote neutrophil degranulation and enhance microbicidal activity of neutrophil trafficking to the intestinal mucosa [103].
Purinergic regulation of secretion from neuroendocrine cells in gastric mucosa
A1/gastrin release
In the stomach, adenosine protects against stress-induced gastric ulcer formation by inhibiting gastric acid secretion by direct action on parietal cells in some species (guinea pigs and dogs, [42, 48]) but not others (rats, [86]). A recent study using selective A1 (N6-cyclopentyladenosine), A2a (CGS 2168), and A3 (IB-MECA) receptor agonists provided pharmacological proof that an A1 receptor is negatively coupled to gastrin release in rat stomach. An A1 receptor selective antagonist 8-cyclopentyl-1,3-dipropyl-xanthine abolished adenosine effects on gastrin release, and A1 immunoreactivity was found on mucosal G cells (gastrin) and D cells (somatostatin), but not parietal cells (H+ ions, acid). Therefore, adenosine may suppress gastrin release by activating A1 receptors on G cells, leading to inhibition of gastric acid secretion [123, 125].
Omeprazole-induced achlorhydria suppresses gene expression of both A1 and A2a receptors in the antrum and corpus mucosa. In the vascularly perfused rat stomach, omeprazole also significantly attenuated the A2a receptor-mediated stimulation of somatostatin release along with a corresponding decrease in A2a receptor mRNA expression. This acid-dependent change in A2a receptor expression is a regulatory feedback mechanism to control gastric acid secretion [124]. The A2a receptor agonist ATL-146e could almost prevent aspirin-induced gastric mucosal lesions [83].
Clinical implications of adenosine deaminase (ADA) activity
Clinical studies suggest that adenosine inhibits gastric acid secretion and may act as a gastroprotective agent. For instance, patients suffering from acid hypersecretion have higher ADA activity. There is a direct correlation between ADA activity and gastric acid output in fundic mucosa of patients with ulcer, gastritis, and achlorhydria [79]. In patients with gastric ulcers, corpus mucosa ADA activity is reduced after H2 blocker ranitidine treatment [80].
A2a receptors and somatostatin release
Adenosine administration to the isolated vascularly perfused rat stomach inhibits gastrin release and stimulates somatostatin release [64], a potent inhibitor of gastric acid secretion. Adenosine analogs augment somatostatin-like immunoreactivity release with a potency profile of CGS 21680 = 5′-N-ethylcarboxamidoadenosine > 2-chlroadenosine > R-(-)-N6-(2-phenylisopropyl)adenosine >1-deoxy-1-[6-[[3-iodophenyl)methyl]amino]-9H-purin-9-yl]-N-methyl-β-D-ribofuranuronamide > N6-cyclopentyladenosine = N6-cyclohexyladenosine > S-(+)-N6-(2-phenylisopropyl) adenosine, suggesting an A2a receptor involvement. The A2a receptor antagonist ZM 241385 abolished the CGS 21680 or adenosine stimulation of somatostatin release. Immunochemical distribution of A2a receptors suggests that adenosine can act on D cells or indirectly on myenteric neurons to stimulate somatostatin release and consequently suppress gastric acid secretion [125].
Gastric preconditioning induced by short ischemia (brief occlusion of celiac artery for 5 min) protects against mucosal damage induced by severe ischemia/reperfusion or topical mucosal irritants in the stomach. Adenosine release among other mediators [63] contributes to protection. Adenosine pretreatment (10 mg/kg i.g.) reduced lesions and improved gastric blood flow similar to ischemic preconditioning. The adenosine receptor antagonist 8-phenyltheophiline (10 mg/kg i.g.) attenuated the gastroprotection due to preconditioning. Furthermore, the selective A2a receptor agonist ATL-146e (5 μg/kg) attenuated stress-induced gastric lesions and damage and reduced production of proinflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-1β] and neutrophil infiltration, suggesting a protective effect via A2 receptors against ulcer formation [82].
Reflex-driven intestinal diarrhea and clinical relevance of purines
The therapeutic potential of purinergic receptors in intestinal secretomotor disorders remains to be established, but evidence is building in support of endogenous adenosine, A2a, and A3 receptors as therapeutic targets. Toxin A (TxA) release from Clostridium difficile (a gram-positive anaerobic bacillus) can contribute to antibiotic-induced diarrhea and pseudomembranous colitis; the latter condition is associated with significant mucosal secretion and inflammation. The neural circuit mechanism deduced from the available evidence is shown in Fig. 9. Briefly, activation of nuclear factor NF-κB initiates a cascade of events involving epithelial cells, macrophages, neutrophils, mast cells, extrinsic primary afferent neurons (EPANs), and the ENS including cholinergic secretomotor neurons. Eventual hyperexcitability of neural circuits leads to amplification of the secretory response and profuse secretion to flush out the pathogenic organism [25]. The selective A2a receptor agonist ATL 313 (0.5-5 nM) also reduced Clostridium difficile TxA-induced secretion and edema, prevented the mucosal damage and neutrophil infiltration, TxA-induced cell death, TNF-α production, as well as increased ileal ADA activity, in murine ileal loops exposed to TxA. Effects were reversed by the selective A2a receptor antagonist ZM241385 (5 nM) [15].
1Clostridium difficile toxin A (TxA) stimulates reflex-evoked intestinal secretion and causes diarrhea. 2 After TxA is internalized in enterocytes, TxA activates NF-kB and releases LTB4, PGE2, TNF. 3 After NF-kB activation, the proinflammatory mediators interleukins (IL) are released from monocytes/macrophages (MO). 4 Stimulation of processes of EPANS occurs, followed by release of substance P (SP)/CGRP that degranulates mast cells (MC) by activating NK1 receptors. 5 SP-containing EPANS release SP on cholinergic secretomotor neurons. The mediators released from mast cells include histamine, 5-HT, PGE2, and tryptase. 6 Tryptase acts on PAR2 receptors on EPANS and on IPANS to stimulate further release of PGE2. 7 The sympathetic innervation with norepinephrine release and its binding to α2-adrenergic receptors normally reduces secretion via VIP secretomotor neurons. ILs suppress norepinephrine release. These cytokines act on sympathetics to lift the sympathetic brake exerted by norepinephrine. TxA-induced release of proinflammatory mediators degranulates mast cells that amplify neural and secretory responses in order to maximize secretion in defense of the host. Treatment with an A2a receptor agonist attenuates the TxA-induced diarrhea presumably by acting in this reflex pathway. (Modified from [25] by permission)
Recent studies provided convincing evidence that activation of an A2a receptor attenuates intestinal inflammation in animal models of IBD and may serve as a novel therapy for IBD [15, 77, 82]. In general, adenosine has diverse functions in the GI tract in the regulation of secretion, motility, innate mucosal immunity, is protective against experimental IBD, and is important in mediating inflammatory responses at sites of injury, and has anti-inflammatory effects in epithelial cells.
Adenosine is also reported to be a conditionally essential nutrient for the gut and has been shown to be beneficial in alleviating severe diarrhea in children with cholera [20, 25, 98].
Recent findings indicate that lumenal adenosine or AMP can rapidly increase glucose transport by small intestine and increase systemic availability of 3-O-methyl glucose after an oral administration to mice. The putative A2 receptor involved is linked to a signaling pathway involving cAMP production. Adenosine causes a rapid increase in carrier-mediated glucose uptake that is suggested to be of clinical relevance [60]. In contrast to other modulators, adenosine is a natural component of the diet, and this alone or as supplement in the diet may suggest potential applications in treatment of malabsorption in chronic intestinal inflammatory diseases. Daily intake of purines is 600-1,000 mg/day by adult individuals in the USA [99]. It has been estimated that diet and endogenous adenosine production can contribute 5-6 mM of adenosine in the lumen in individuals on a Western diet. A concentration of 1 mM luminal adenosine caused a maximum effect on glucose uptake [60].
In human intestinal epithelial cells, adenosine affects immune function and exerts anti-inflammatory effects by acting as a negative regulator of NF-κB and MAPK signaling. Adenosine pretreatment in HT-29 cells caused a reduction in stimulated IL-8 expression and secretion [55].
Neurogenic diarrhea
The adenosine A3 receptor is one of the least understood receptors in secretomotor reflexes. The physiological relevance of A3 receptor is unclear, and current opinion is that it may function primarily in disease or abnormal states such as ischemia/reperfusion injury, tissue damage, gut inflammation, and extremes in motor activity of the gut when endogenous adenosine release is sufficiently high to activate the low affinity receptor [53]. It has been reported by Cooke et al. [30] that dimaprit–H2R activation of submucous neurons leads to a stereotypical cyclical pattern of secretion in coordination with motility that can last for several hours. The underlying electrophysiological correlate is cyclical depolarization and excitation of submucous neurons. This is a model of neurogenic diarrhea involving an immune-neural circuit. It proved useful in elucidating a functional role of A3 receptors in addition to A1 receptors in the secretomotor function and coordination of motility and secretion [8, 9]. Dimaprit was used to elicit a stereotypical cyclical increase in short-circuit current (Isc = chloride secretion) in coordination with motility. Isc was recorded in guinea pig distal colon simultaneously with muscle length in the circular orientation by sonomicrometry. A1 receptor-selective antagonists 8-cyclopentyltheophylline (CPT) or 1,3-dipropyl-8-(2-amino-4-chlorophenyl) xanthine (PACPX) or the irreversible antagonist FSCPX caused a concentration-dependent augmentation of coordinated responses; 100 nM antagonist caused maximum blockade of A1 receptors and enhanced Isc 133% and ICD 64% with ED50 values in the nanomolar concentration range. Dimaprit responses were abolished by 10 nM A1 receptor agonist CCPA and reversed by A1 receptor antagonists. After knockdown of A1 receptors with FSCPX (1 μM), the selective A3 receptor agonist IB-MECA could still abolish coordinated responses (IC50-Isc = 2.1 μM; IC50-ICD = 7.1 μM) that could be reversed by the A3 receptor antagonist MRS1191. The A3 receptor antagonist alone could also enhance Isc and coordinated muscle responses. The coordinated response to dimaprit is abolished by nerve blockade with TTX, and remaining myogenic activity was reduced by ~50% by IB-MECA after A1 receptor knockdown with FSCPX. MRS1191 (1 μM) could enhance TTX-sensitive distension-evoked Isc responses. A3 receptor immunoreactivity occurs in myenteric and submucous neurons of guinea pig, mouse, rat, and human colon (Christofi, unpublished observations). Availability of knockout models for adenosine receptor subtypes should provide unequivocal proof of function.
In summary, preliminary data suggest that in a model of neurogenic diarrhea endogenous adenosine release is sufficient to act at inhibitory A1 and A3 receptors to modulate the stereotype neural-motor behavior triggered by the mast cell mediator histamine. Adenosine A3 receptors may be targets for modulation of distension-evoked neurosecretory reflexes as well. The role of A3 receptors in bowel disorders associated with diarrhea or diarrhea predominant IBS warrants further investigation.
Differential dysregulation of purinoceptors in GI disease states
As already described, purinergic signaling regulates all important physiological functions of the GI tract including epithelial transport, 5-HT release from EC cells, mucosal reflexes, sensory signaling in the ENS, synaptic transmission, as well as coordination of motility and secretion. It is reasonable then to suggest that these abnormalities in purinergic receptors or signaling pathways would have a significant impact on the overall behavior of the gut, enteric reflexes, and secretomotor behavior of the gut in bowel diseases. Purinergic receptors in gastrointestinal inflammation were the subject of a recent review by Kolachala et al. [61]. A recent study by Guzman et al. [45] using high-density oligonucleotide microarray analysis and SYBR-Green RT-PCR validation provided direct proof for differential dysregulation (upregulation or downregulation depending on the purine gene) of P2X1, P2X2, P2X4, P2X7, P2Y1, P2Y2, P2Y4, P2Y6, A2a, A2b, A1, or A3 receptors in whole thickness gut specimens of experimental trinitrobenzene sulfonic acid (TNBS) colitis in rats . Expression of A1 and A3 receptor gene products is also altered in a chronic ileitis model of Crohn’s disease [106]. The ATP-gated ion channel P2X3 is increased in human IBD [122] as is the case for TNBS colitis [45]. In submucosal neurons of TNBS colitis, there is emergence of a purinergic component of the fast EPSP that is cholinergic in nature in normal animals [69, 70]. Therefore, there is plasticity of purinergic neurotransmission. Abnormalities in the neural regulation of the gastrointestinal vasculature could contribute to the pathogenesis of IBD. Recent data suggest that reduced purinergic neurotransmission via putative P2X1 receptors may underlie a defect in sympathetic regulation of gut vasomotor function during TNBS colitis [68]. Changes in vasomotor function could also impact on secretomotor responses. Further studies are needed to identify the location of these receptors in the gut wall and better understand the functional relevance of these and other receptor abnormalities on secretomotor and vasomotor function in various species and in human gut.
It was recently shown that purinergic gene dysregulation in experimental colitis is sensitive to oral administration of the A3 receptor agonist IB-MECA, a potential therapeutic target for IBD [45]. IB-MECA protects against tissue injury, development of colitis, and prevents dysregulation of 92% of genes in TNBS colitis, including purine genes. This illustrates a new important benefit of A3 receptor agonists in preventing abnormalities in purine gene expression in colitis. A review by Antonioli et al. [2] discusses the pharmacological modulation of adenosinergic pathways in the therapeutic management of IBD. Adenosine A1, A2a, A2b, and A3 receptors are potential therapeutic targets as regulators of gut enteric immune responses and all components of gut motor reflexes [2, 27].
Summary and conclusions
A secretomotor reflex is activated by mucosal stroking, distension, or chemical stimulation of the mucosa leading to release of 5-HT or other mediators, subsequent activation of IPANs in synaptic communication with interneurons or secretomotor neurons to stimulate chloride and fluid secretion. Inputs from myenteric neurons modulate secretory rates and reflexes, and special neural circuits exist to coordinate secretion with motility and vasomotor functions. All cellular components of secretomotor reflexes express multiple purinergic receptors for adenosine or the nucleotides ATP, ADP, UTP, or UDP. This review focused on the emerging concepts in our understanding of purinergic regulation at these receptors, and in particular of mechanosensory reflexes. Endogenous purines can activate inhibitory A1, A3, and P2Y12 receptors or stimulatory A2 and P2Y1 receptors to modulate 5-HT release. The resting “secretory tone” is influenced by endogenous purines. Stimulatory P2Y1 receptor or inhibitory A1/A3 receptors are involved in mechanically evoked secretomotor reflexes and a model of neurogenic diarrhea. Recent studies identified the neural and/or non-neural distribution profiles of P2X2, P2X3, P2X5, P2Y1, P2Y2, P2Y4, P2Y6, or P2Y12 receptor immunoreactivity in the GI tract suggesting their role in secretomotor function. Significant species and regional differences exist in distribution and function of the receptors. Abnormal expression of purinergic receptors may contribute to functional abnormalities and diarrhea in IBD; functional studies are lacking. Adenosine A2a or A3 receptors are emerging as therapeutic targets in IBD and protect against purinergic receptor abnormalities or profuse diarrhea. Purines are emerging as fundamental regulators of enteric secretomotor reflexes in health and disease.
Future studies should be aimed at better understanding the function of other purinergic receptors expressed in the enteric nervous system or non-neuronal components of the reflex, in both rodents and human gut. More integrative studies on purinergic regulation of chemosensory or mechanosensory secretomotor reflexes are desperately needed. This should pave the way to better understand purine receptor biology, abnormalities in disease states, or as therapeutic targets.
Abbreviations
- 5-HT:
-
serotonin
- ADA:
-
adenosine deaminase
- AK:
-
adenosine kinase
- AR-C69931MX or cangrelor:
-
N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-βγ-dichloromethylene-ATP
- ATP:
-
adenosine 5′-triphosphate
- CaCC:
-
Ca2+-dependent Cl- channels
- cAMP:
-
3′,5′-cyclic adenosine monophosphate
- CFTR:
-
cystic fibrosis conductance transmembrane regulator
- ChaT:
-
choline acetyltransferase
- EC:
-
enterochromaffin cell
- ENaC:
-
amiloride-sensitive epithelial Na+ channels
- ENS:
-
enteric nervous system
- EPAN:
-
extrinsic primary afferent neuron
- GI:
-
gastrointestinal tract
- IBD:
-
inflammatory bowel diseases
- IPAN:
-
intrinsic primary afferent neuron
- -ir:
-
immunoreactivity
- Isc:
-
short-circuit current indicative of chloride secretion
- 2MeSADP:
-
2-methylthioADP
- MRS2279:
-
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′5′-bisphosphate
- MRS2179:
-
2′-deoxy-N6-methyladenosine-3′5′-bisphosphate
- MRS2211:
-
6-(2′-chloro-5-nitro-azophenyl)-pyridoxal-α5-phosphate
- MRS2567:
-
1.2-di-(4-isothio-cyanatophenyl)ethane
- NBTI:
-
S-(p-nitrobenzyl)-6-thioinosine
- NPY:
-
neuropeptide Y
- PLC:
-
phospholipase C
- PPADS:
-
pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt
- R:
-
receptor
- SP:
-
substance P
- VIP:
-
vasoactive intestinal peptide
References
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58(3):281–341
Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C (2008) Pharmacological modulation of adenosine system: novel options for treatment of inflammatory bowel diseases. Inflamm Bowel Dis 14:566–574
Barajas-Lopez C, Espinosa-Luna R, Christofi FL (2000) Changes in intracellular Ca2+ by activation of P2 receptors in submucosal neurons in short-term cultures. Eur J Pharmacol 409(3):243–257
Bearcroft CP, Perrett D, Farthing MJ (1998) Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 42(1):42–46
Bertrand PP (2006) Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol 577(Pt 2):689–704
Bertrand PP (2003) ATP and sensory transduction in the enteric nervous system. Neuroscientist 9(4):243–260
Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ (2003) Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol 551(Pt 1):309–322
Boserov A, Wang YZ, Javed N, Christofi FL, Cooke HJ (2005) Comparison of adenosine A1 receptor activation on repetitive reflex Cl secretion coordinated with repetitive muscle activity measured by sonomicrometry and force transducer tension. Gastroenterology 128(4 Suppl 2):A–608
Bozarov A, Wang Y-Z, Yu JG, Cooke HJ, Christofi FL (2006) Endogenous adenosine acts at A1 or A3 inhibitory receptors to modulate the stereotype neural-motor behavior of the gut triggered by histamine or distension. Gastroenterology 130(4 Suppl 2):A–29
Brookes SJ, Steele PA, Costa M (1991) Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience 42(3):863–878
Brookes SJ (2001) Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262(1):58–70
Burnstock G (2006) Purinergic signalling. Br J Pharmacol 147(Suppl 1):S172–S181
Cabarrocas J, Savidge TC, Liblau RS (2003) Role of enteric glial cells in inflammatory bowel disease. Glia 41(1):81–93
Castelucci P, Robbins HL, Poole DP, Furness JB (2002) The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol 117(5):415–422
Cavalcante IC, Castro MV, Barreto AR, Sullivan GW, Vale M, Almeida PR, Linden J, Rieger JM, Cunha FQ, Guerrant RL, Ribeiro RA, Brito GA (2006) Effect of novel A2A adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect Immun 74(5):2606–2612
Chan HC, Cheung WT, Leung PY, Wu LJ, Chew SB, Ko WH, Wong PY (1996) Purinergic regulation of anion secretion by cystic fibrosis pancreatic duct cells. Am J Physiol 271(2 Pt 1):C469–C477
Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel AJ, Javed NH, Yu JG, Grants I, Cooke HJ (2004) Endogenous adenosine differentially modulates 5-hydroxytryptamine from a human enterochromaffin cell model. Gastroenterology 127:188–202
Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, Javed N, Cooke H (2004) Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol 469(1):16–36
Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ (2001) Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol 439(1):46–64
Christofi FL (2001) Unlocking mysteries of gut sensory transmission: is adenosine the key? News Physiol Sci 16:201–207
Christofi FL, Wood JD (1993) Presynaptic inhibition by adenosine A1 receptors on guinea pig small intestinal myenteric neurons. Gastroenterology 104(5):1420–1429
Christofi FL, Tack J, Wood JD (1992) Suppression of nicotinic synaptic transmission by adenosine in myenteric ganglia of the guinea-pig gastric antrum. Eur J Pharmacol 216(1):17–22
Clarke LL, Harline MC, Gawenis LR, Walker NM, Turner JT, Weisman GA (2000) Extracellular UTP stimulates electrogenic bicarbonate secretion across CFTR knockout gallbladder epithelium. Am J Physiol Gastrointest Liver Physiol 279(1):G132–G138
Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL (2004) Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126(7):1657–1664
Cooke HJ, Christofi FL (2006) Enteric neural regulation of mucosal secretion. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 4th edn. Academic, New York, pp 737–762
Cooke HJ, Wunderlich J, Christofi FL (2003) “The force be with you”: ATP in gut mechanosensory transduction. News Physiol Sci 18:43–49
Cooke HJ, Xue J, Yu JG, Wunderlich J, Wang YZ, Guzman J, Javed N, Christofi FL (2004) Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol 469(1):1–15
Cooke HJ, Javed N, Christofi FL (2003) Enteric neural reflexes and secretion. In: Bienenstock J, Goetzl EJ, Blennerhassett MG (eds) Autonomic neuroimmunology, 1st edn. CRC Press, Boca Raton, pp 35–59
Cooke HJ, Wang Y, Liu CY, Zhang H, Christofi FL (1999) Activation of neuronal adenosine A1 receptors suppresses secretory reflexes in the guinea pig colon. Am J Physiol 276(2 Pt 1):G451–G462
Cooke HJ, Wang YZ, Rogers R (1993) Coordination of Cl- secretion and contraction by a histamine H2-receptor agonist in guinea pig distal colon. Am J Physiol 265(5 Pt 1):G973–G978
Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G (2005) P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol 288(5):G1024–G1035
Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR (1999) Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(-) transport. J Biol Chem 274(37):26461–26468
Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198(5):783–796
Evers BM, Ishizuka J, Townsend CM Jr, Thompson JC (1994) The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci 733:393–406
Evers BM, Townsend CM Jr, Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC (1991) Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 101(2):303–311
Facer P, Knowles CH, Tam PK, Ford AP, Dyer N, Baecker PA, Anand P (2001) Novel capsaicin (VR1) and purinergic (P2X3) receptors in Hirschsprung’s intestine. J Pediatr Surg 36(11):1679–1684
Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, Xia Y, Wood JD (2006) Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol 536(1-2):113–122
Furness JB, Kunze WA, Clerc N (1999) Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol 277(5 Pt 1):G922–G928
Furness JB (2000) Types of neurons in the enteric nervous system. J Auton Nerv Syst 81(1-3):87–96
Galligan JJ (2004) Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol 141:1294–1302
Gao N, Hu HZ, Zhu MX, Fang X, Liu S, Gao C, Wood JD (2006) The P2Y purinergic receptor expressed by enteric neurones in guinea-pig intestine. Neurogastroenterol Motil 18(4):316–323
Gerber JG, Payne NA (1988) Endogenous adenosine modulates gastric acid secretion to histamine in canine parietal cells. J Pharmacol Exp Ther 244(1):190–194
Groschel-Stewart U, Bardini M, Robson T, Burnstock G (1999) P2X receptors in the rat duodenal villus. Cell Tissue Res 297(1):111–117
Gustafsson BI, Bakke I, Tommeras K, Waldum HL (2006) A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand J Gastroenterol 41:390–395
Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL (2006) ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis 12(8):766–789
Gwynne RM, Bornstein JC (2007) Local inhibitory reflexes excited by mucosal application of nutrient amino acids in guinea pig jejunum. Am J Physiol Gastrointest Liver Physiol 292(6):G1660–G1670
Hede SE, Amstrup J, Christoffersen BC, Novak I (1999) Purinoceptors evoke different electrophysiological responses in pancreatic ducts. P2Y inhibits K(+) conductance, and P2X stimulates cation conductance. J Biol Chem 274(45):31784–31791
Heldsinger AA, Vinik AI, Fox IH (1986) Inhibition of guinea-pig oxyntic cell function by adenosine and prostaglandins. J Pharmacol Exp Ther 237(2):351–356
Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD (2001) P2X(7) receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol 440(3):299–310
Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD (2003) Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550(Pt 2):493–504
Hubel KA (1978) The effects of electrical field stimulation and tetrodotoxin on ion transport by the isolated rabbit ileum. J Clin Invest 62(5):1039–1047
Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, Steward MC (1999) Luminal ATP stimulates fluid and HCO3- secretion in guinea-pig pancreatic duct. J Physiol 519(Pt 2):551–558
Jacobson KA, Gao ZG (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5(3):247–264
Jenkinson KM, Reid JJ (2000) The P(2)-purinoceptor antagonist suramin is a competitive antagonist at vasoactive intestinal peptide receptors in the rat gastric fundus. Br J Pharmacol 130(7):1632–1638
Jijon HB, Walker J, Hoentjen F, Diaz H, Ewaschuk J, Jobin C, Madsen KL (2005) Adenosine is a negative regulator of NF-kappaB and MAPK signaling in human intestinal epithelial cells. Cell Immunol 237(2):86–95
Kerstan D, Gordjani N, Nitschke R, Greger R, Leipziger J (1998) Luminal ATP induces K+ secretion via a P2Y2 receptor in rat distal colonic mucosa. Pflugers Arch 436(5):712–716
Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP (2001) International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev 53(1):107–118
Kidd M, Modlin IM, Eick GN, Champaneria MC (2006) Isolation, functional characterization, and transcriptome of Mastomys ileal enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol 291(5):G778–G791
Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE (2001) D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology 121(6):1400–1406
Kimura Y, Turner JR, Braasch DA, Buddington RK (2005) Lumenal adenosine and AMP rapidly increase glucose transport by intact small intestine. Am J Physiol Gastrointest Liver Physiol 289(6):G1007–G1014
Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV (2008) Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol 294(2):G401–G410
Konig J, Schreiber R, Mall M, Kunzelmann K (2002) No evidence for inhibition of ENaC through CFTR-mediated release of ATP. Biochim Biophys Acta 1565(1):17–28
Konturek SJ, Brzozowski T, Pajdo R, Konturek PC, Kwiecien S, Sliwowski Z, Pawlik M, Ptak A, Drozdowicz D, Hahn EG (2001) Gastric preconditioning induced by short ischemia: the role of prostaglandins, nitric oxide and adenosine. Med Sci Monit 7(4):610–621
Kwok YN, McIntosh C, Brown J (1990) Augmentation of release of gastric somatostatin-like immunoreactivity by adenosine, adenosine triphosphate and their analogs. J Pharmacol Exp Ther 255(2):781–788
Lazarowski ER, Rochelle LG, O’Neal WK, Ribeiro CM, Grubb BR, Zhang V, Harden TK, Boucher RC (2001) Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. J Pharmacol Exp Ther 297(1):43–49
Lee MG, Schultheis PJ, Yan M, Shull GE, Bookstein C, Chang E, Tse M, Donowitz M, Park K, Muallem S (1998) Membrane-limited expression and regulation of Na+-H+ exchanger isoforms by P2 receptors in the rat submandibular gland duct. J Physiol 513(Pt 2):341–357
Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM (2003) Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 285(1):G207–G216
Lomax AE, O’Reilly M, Neshat S, Vanner SJ (2007) Sympathetic vasoconstrictor regulation of mouse colonic submucosal arterioles is altered in experimental colitis. J Physiol 583(Pt 2):719–730
Lomax AE, Linden DR, Mawe GM, Sharkey KA (2006) Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci 126–127:250–257
Lomax AE, Fernández E, Sharkey KA (2005) Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil 17(1):4–15
Lomax AE, Furness JB (2000) Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res 302(1):59–72
Luo X, Zheng W, Yan M, Lee MG, Muallem S (1999) Multiple functional P2X and P2Y receptors in the luminal and basolateral membranes of pancreatic duct cells. Am J Physiol 277(2 Pt 1):C205–C215
Madara JL, Nash S, Parkos C (1991) Neutrophil-epithelial cell interactions in the intestine. Adv Exp Med Biol 314:329–334
Matos JE, Sorensen MV, Geyti CS, Robaye B, Boeynaems JM, Leipziger J (2007) Distal colonic Na(+) absorption inhibited by luminal P2Y(2) receptors. Pflugers Arch 454(6):977–987
Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J (2005) K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol 564(Pt 1):269–279
McAlroy HL, Ahmed S, Day SM, Baines DL, Wong HY, Yip CY, Ko WH, Wilson SM, Collett A (2000) Multiple P2Y receptor subtypes in the apical membranes of polarized epithelial cells. Br J Pharmacol 131(8):1651–1658
Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB (2006) Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177(5):2765–2769
Nahum V, Zundorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B (2002) Adenosine 5′-O (1-boranotriphosphate) derivatives as novel P2Y(1) receptor agonists. J Med Chem 45(24):5384–5396
Namiot Z, Rutkiewicz J, Stasiewicz J, Gorski J (1990) Adenosine deaminase activity in the human gastric mucosa in relation to acid secretion. Digestion 45(3):172–175
Namiot Z, Rutkiewicz J, Stasiewicz J, Baranczuk E, Marcinkiewicz M (1991) Adenosine deaminase activity in the gastric mucosa in patients with gastric ulcer. Effects of ranitidine and sucralfate. Eur J Pharmacol 205(1):101–103
Nguyen TD, Moody MW, Savard CE, Lee SP (1998) Secretory effects of ATP on nontransformed dog pancreatic duct epithelial cells. Am J Physiol 275(1 Pt 1):G104–G113
Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F (2005) Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129(1):26–33
Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Horikawa Y, Matsuhashi T, Hatakeyama N, Oyake J, Ohba R, Watanabe S, Linden J (2006) Attenuation of gastric mucosal inflammation induced by aspirin through activation of A2A adenosine receptor in rats. World J Gastroenterol 12(4):568–573
Pan H, Gershon MD (2000) Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci 20(9):3295–3309
Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB (2002) The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci 101(1-2):39–47
Puurunen J, Ruoff HJ, Schwabe U (1987) Lack of direct effect of adenosine on the parietal cell function in the rat. Pharmacol Toxicol 60(4):315–317
Raybould HE, Cooke HJ, Christofi FL (2004) Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil 16(Suppl 1):60–63
Reed DE, Vanner S (2007) Mucosal stimulation activates secretomotor neurons via long myenteric pathways in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 292(2):G608–G614
Rivkees SA, Reppert SM (1992) RFL9 encodes an A2b-adenosine receptor. Mol Endocrinol 6(10):1598–1604
Robaye B, Ghanem E, Wilkin F, Fokan D, Van Driessche W, Schurmans S, Boeynaems JM, Beauwens R (2003) Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol Pharmacol 63(4):777–783
Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA (2005) Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 25(27):6286–6295
Roman RM, Fitz JG (1999) Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology 116(4):964–979
Ruan HZ, Burnstock G (2004) P2X2 and P2X3 receptor expression in the gallbladder of the guinea pig. Auton Neurosci 111(2):89–96
Ruan HZ, Burnstock G (2005) The distribution of P2X5 purinergic receptors in the enteric nervous system of mouse. Cell Tissue Res 319(2):191–200
Ruhl A, Nasser Y, Sharkey KA (2004) Enteric glia. Neurogastroenterol Motil 16(Suppl 1):44–49
Salter KD, Fitz JG, Roman RM (2000) Domain-specific purinergic signaling in polarized rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 278(3):G492–G500
Saslow SB, Scolapio JS, Camilleri M, Forstrom LA, Thomforde GM, Burton DD, Rubin J, Pitot HC, Zinsmeister AR (1998) Medium-term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut 42(5):628–634
Sanchez-Pozo A, Gil A (2002) Nucleotides as semiessential nutritional components. Br J Nutr 87(Suppl 1):S135–S137
Sarwar G, Brule D (1991) Assessment of the uricogenic potential of processed foods based on the nature and quantity of dietary purines. Prog Food Nutr Sci 15(3):159–181
Schlenker T, Romac JM, Sharara AI, Roman RM, Kim SJ, LaRusso N, Liddle RA, Fitz JG (1997) Regulation of biliary secretion through apical purinergic receptors in cultured rat cholangiocytes. Am J Physiol 273(5 Pt 1):G1108–G1117
Schreiber R, Konig J, Sun J, Markovich D, Kunzelmann K (2003) Effects of purinergic stimulation, CFTR and osmotic stress on amiloride-sensitive Na+ transport in epithelia and Xenopus oocytes. J Membr Biol 192(2):101–110
Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB (1995) CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81(7):1063–1073
Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL (2001) Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 107(7):861–869
Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL (1997) Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest 99(11):2588–2601
Suarez-Huerta N, Pouillon V, Boeynaems J, Robaye B (2001) Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur J Pharmacol 416(3):197–202
Sundaram U, Hassanain H, Suntres Z, Yu JG, Cooke HJ, Guzman J, Christofi FL (2003) Rabbit chronic ileitis leads to up-regulation of adenosine A1/A3 gene products, oxidative stress, and immune modulation. Biochem Pharmacol 65(9):1529–1538
Vank C, Fromter E, Kottra G (1999) The activation of an apical Cl- conductance by extracellular ATP is potentiated by genistein in Necturus gallbladder epithelium. Pflugers Arch 438(4):497–501
Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans JP (2002) Neurochemical identification of enteric neurons expressing P2X(3) receptors in the guinea-pig ileum. Histochem Cell Biol 118(3):193–203
Van Nassauw L, Costagliola A, Van Op den Bosch J, Cecio A, Vanderwinden JM, Burnstock G, Timmermans JP (2006) Region-specific distribution of the P2Y4 receptor inenteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci 126-127:299–306
von Kugelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110(3):415–432
Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R (1996) Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A 93(15):8063–8067
Weber E, Neunlist M, Schemann M, Frieling T (2001) Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol 536(Pt 3):741–751
Wood JD (2006) The enteric purinergic P2Y(1) receptor. Curr Opin Pharmacol 6(6):564–570
Wunderlich J, Xue J, Cooke HJ, Christofi FL (2004) Visualization and mapping of mechanosensitive P2X receptors in the human BON–enterochromaffin cell model. Gastroenterology 126(Suppl 2):A–412
Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang YZ, Grants I, Mikami D, Melvin WS, Cooke HJ, Christofi FL (2008) Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294(2):G554–G566
Wynn G, Rong W, Xiang Z, Burnstock G (2003) Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology 125(5):1398–1409
Xiang Z, Burnstock G (2006) Distribution of P2Y(6) and P2Y(12) receptor: their colocalization with calbindin, calretinin and nitric oxide synthase in the guinea pig enteric nervous system. Histochem Cell Biol 125(4):327–336
Xiang Z, Burnstock G (2005) Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol 124(5):379–390
Xiang Z, Burnstock G (2004) P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol 121(3):169–179
Xue J Christofi FL, Cooke HJ (2007) P2Y12 receptors inhibit mechanosensitive release of 5-HT from human carcinoid BON cells. Gastroenterology 132:A547
Xue J, Christofi FL, Cooke HJ (2007) Mechanosensitive release of calcium or 5HT: P2Y1 receptor over-expression in human Bon cells. Gastroenterology 132(Suppl 2):A–242
Yiangou Y, Facer P, Baecker PA, Ford AP, Knowles CH, Chan CL, Williams NS, Anand P (2001) ATP-gated ion channel P2X(3) is increased in human inflammatory bowel disease. Neurogastroenterol Motil 13(4):365–369
Yip L, Kwok YN (2004) Role of adenosine A2A receptor in the regulation of gastric somatostatin release. J Pharmacol Exp Ther 309(2):804–815
Yip L, Leung HC, Kwok YN (2004) Effect of omeprazole on gastric adenosine A1 and A2A receptor gene expression and function. J Pharmacol Exp Ther 311(1):180–189
Yip L, Leung HC, Kwok YN (2004) Role of adenosine A1 receptor in the regulation of gastrin release. J Pharmacol Exp Ther 310(2):477–487
Acknowledgements
Many thanks to Drs. Helen J. Cooke, Jacqueline Wunderlich, Jun Ge Yu, Shelly Xue, Jorge Guzman, Huiming Zheng, Asad Javed, and Iveta Grants for their research contributions to the overall field of study. Some cited studies and this review were supported by the National Institutes of Health (NIH) grant numbers R01 DK44179 years 1–13, a 3 year Fellowship to Dr Jorge Guzman and NCRR 1S10RR11434 and OSU-seed funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Christofi, F.L. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signalling 4, 213–236 (2008). https://doi.org/10.1007/s11302-008-9104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-008-9104-4