Abstract
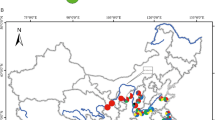
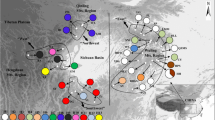
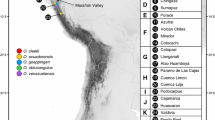
Genetic variation, haplotype relationships, and potential regions of diversity in Symplocos laurina Wall. (Symplocaceae), a montane tree species from India, have been tested using molecular and biogeographical data to infer phylogeographic patterns. The polymerase chain reaction-restriction fragment length polymorphism technique was used to determine the chloroplast (cp) and mitochondrial (mt) DNA haplotypes of 218 individuals from 12 populations, collected from Northeast India (NEI), which is a part of Indo-Burma biodiversity hotspot, and South India, which includes the Western Ghats (WG), another biodiversity hotspot from India and Eastern Ghats (EG). Nine cpDNA (chlorotypes) and 24 mtDNA haplotypes (mitotypes) were identified; the WG region was identified as the most diverse for chlorotypes and the NEI region for mitotypes. Although a strong population differentiation was observed, phylogeographic structure was found to be absent for both the genomes. The haplotype network revealed the presence of two main lineages: NEI-WG lineage and EG lineage, largely without overlapping distributions. The study tests two hypotheses, namely vicariance and dispersal, to understand the distribution of plants in India. The population structure of S. laurina strongly suggests the persistence of the species in putative refugial areas preglaciation and further establishment of other populations of S. laurina from these refugial populations.



Similar content being viewed by others
References
Almeida SM (1990) The flora of Savantwadi, Maharashtra, India. Scientific Publishers, Jodhpur
Apte GS, Bahulikar RA, Kulkarni RS et al (2006) Genetic diversity analysis in Gaultheria fragrantissima Wall. (Ericaceae) from the two biodiversity hotspots in India using ISSR markers. Curr Sci 91:1634–1640
Auden JB (1949) A geological discussion on the Satpura hypothesis and Garo-Rajmahal gap. Proc Natl Acad Sci U S A 8:315–340
Bahulikar RA, Lagu MD, Kulkarni BG et al (2004) Genetic diversity among spatially isolated populations of Eurya nitida Korth. (Theaceae) based on inter-simple sequence repeats. Curr Sci 86:824–831
Bande MB, Chandra S (1990) Early tertiary vegetational reconstructions around Nagpur-Chhindwara and Mandla, central India. Palaeobotanist 38:196–208
Blasco F (1970) Aspects of flora and ecology of savannas of the South Indian hills. J Bombay Nat Hist Soc 67:522–534
Blasco F (1971) Orophytes of South India and Himalayas. J Indian Bot Soc 50:377–381
Burban C, Petit RJ, Caracreff E et al (1999) Rangewide variation of the maritime pine bast scale Matsococcus feytauti Duc. (Homoptera: Matsucoccidae) in relation to the genetic structure of its host. Mol Ecol 8:1593–1602
Chauhan MS (2002) Holocene vegetation and climatic changes in southeastern Madhya Pradesh, India. Curr Sci 83:1444–1445
Conservation International (2005) Biodiversity Hotspots Conservation International, Washington, DC. http://www.biodiversityhotspots.org/xp/Hotspots/
Daniels RJR (1992) Geographical distribution patterns of amphibians in the Western Ghats, India. J Biogeogr 19:521–529
Demesure B, Sodzi N, Petit RJ (1995) A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Mol Ecol 4:129–131
Deshpande AU, Apte GS, Bahulikar RA et al (2001) Genetic diversity across the natural populations of three montane plant species from the WG, India revealed by ISSR repeats. Mol Ecol 10:2397–2408
Desplanque B, Viard F, Bernard J et al (2000) The linkage disequilibrium between chloroplast DNA and mitochondrial DNA haplotypes in Beta vulgaris ssp. Maritime (L.): the usefulness of both genomes for population genetics studies. Mol Ecol 9:141–154
Dilger WC (1952) The Brij hypothesis as an explanation for the tropical faunal similarities between the Western ghats and the eastern Himalayas, Assam, Burma, and Malaya. Evolution 6:125–127
Dumolin-Lapègue S, Demesure B, Fineshi S et al (1997a) Phylogenetic structure of white oaks throughout the European continent. Genetics 146:1475–1487
Dumolin-Lapègue S, Pemonge M-H, Petit RJ (1997b) An enlarged set of consensus primers for the study of organelle DNA in plants. Mol Ecol 6:393–397
Dumolin-Lapègue S, Pemonge M-H, Petit RJ (1998) Association between chloroplast and mitochondrial lineages in oaks. Mol Biol Evol 15:1321–1331
El Mousadik A, Petit RJ (1996) Chloroplast DNA phylogeography of the argan tree of Morocco. Mol Ecol 5:547–555
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Grivet D, Petit RJ (2002) Phylogeography of the common ivy (Hedera sp.) in Europe: genetic differentiation through space and time. Mol Ecol 11:1351–1362
Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol Ecol 8:521–523
Hampe A, Arroya J, Jordano P, Petit RJ (2003) Rangewide phylogeography of a bird-dispersed Eurasian shub: contrasting Mediterranean and temperate glacial refugia. Mol Ecol 12:3415–3426
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hewitt GM (2000) The genetic legacy of the Quaternary iceages. Nature 405:907–913
Hora SL (1949) Satpura hypothesis of the distribution of the Malayan fauna and flora to peninsular India. Proc Natl Inst Sci India 15:309–314
Jain A, Pandit MK, Elahi S et al (2000) Reproductive behaviour and genetic variability in geographically isolated populations of Rhododendron arboreum (Ericaceae). Curr Sci 79:1377–1381
Jaramillo-Correa JP, Beaulieu J, Bousquet J (2004) Variation in mitochondrial DNA reveals multiple distant glacial refugia in black spruce (Picea mariana), a trans continental North American conifer. Mol Ecol 13:1735–2747
Karanth PK (2003) Evolution of disjunct distribution among wet-zone species of the Indian subcontinent: testing various hypotheses using a phylogenetic approach. Curr Sci 85:101–108
Latta RG, Mitton JB (1997) A comparison of population differentiation across four classes of gene marker in Limber pine (Pinus flexilis James). Genetics 146:1153–1163
Ledig FT, Hodgskiss PD, Jacob-Cervantes V (2002) Genetic diversity, mating system, and conservation of a Mexican subalpine relict, Picea mexicana Martínez. Conserv Genet 3:113–122
Legris P, Meher-Homji VM (1984) The Eastern Ghats: phytogeographic aspects. Ind Rev Life Sci 4:115–136
Liedloff A (1999) A Mantel nonparametric test calculator for windows version 2.00. Tropical Ecosystems Research Centre, Winnellie, Northern Territory, Australia
Meher-Homji VM (1967) Phytogeography of the South Indian hill stations. Bull Torrey Bot Club 94:230–242
Meher-Homji VM (1972) Himalayan plants on South Indian Hills: role of Pleistocene glaciation vs long distance dispersal. Sci Cult 38:8–12
Meher-Homji VM (1975) On the montane species of Kodaikanal, South India. Phytocoenologia 2:28–39
Nei M, Chesser RK (1983) Estimation of fixation indices and gene diversities. Ann Hum Genet 47:253–259
Page RD (1996) Treeview: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Palmé AE, Su Q, Rautenberg A et al (2003) Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Mol Ecol 12:201–212
Pons O, Petit RJ (1995) Estimation, variance and optimal sampling of gene diversity. I. Haploid locus. Theor Appl Genet 90:462–470
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Randhwa MS (1945) Progressive desiccation of northern India in historical times. J Bombay Nat Hist Soc 45:558–565
Rhoads DD, Roufa DJ (1991) SEQAID II, version 3.81. Molecular Genetics Laboratory, Kansas State University, Kansas
Richards E, Reichardt M, Rogers S (1994) Preparation of genomic DNA from plant tissues. In: Ausubel EM et al (eds) Current protocols in molecular biology. Wiley, New York, pp 2.3.1–2.3.7
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Templeton AR, Routman E, Phillips CA (1995) Separating population structure from population history: a cladistic analysis of the geographical distribution of mitochondrial DNA sequence data III. Cladogram estimation. Genetics 140:767–782
Tzedakis PC, Lawson IT, Forgley MR, Hewitt GM (2002) Buffered tree population changes in a Quaternary refugium: evolutionary implications. Science 297:2044–2047
Van Rossum F, Bonnin I, Fénart S et al (2004) Spatial genetic structure within a metallicolous population of Arabidopsis halleri, a clonal, self-incompatible and heavy metal-tolerant species. Mol Ecol 13:2959–2967
Vishnu-Mittre, Gupta HP (1970) The origin of Shola forest in the Nilgiris, South India. Paleobotanist 19:110–114
Wright S (1931) Evolution in mendelian populations. Genetics 16:97–159
Acknowledgements
The authors thank Ram Kulkarni and Rasika Bhagawat from the National Chemical Laboratory, Dr. B. G. Kulkarni and Dr. P. S. N. Rao from the Botanical Survey of India, and H. S. Suresh from the Centre for Ecological Science, Indian Institute of Science, Bangalore, for the help rendered during sampling. We also thank the staff at the various field stations and Forest Departments for their cooperation in sampling. We thank Dr. Anargha Wakhare (Department of Geography, Nowrosjee Wadia College, Pune) for her insightful suggestions and help in preparing the map. We thank Dr. Rebecca Zwart from the National Chemical Laboratory, for her help in refining the manuscript. SB thanks the Council of Scientific and Industrial Research, New Delhi, for her research fellowship. This project was supported by a grant from the Department of Biotechnology, New Delhi, to NCL and BSI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Dandekar
Rights and permissions
About this article
Cite this article
Banu, S., Lagu, M.D. & Gupta, V.S. Phylogeographical studies in disjunct populations of Symplocos laurina Wall. using cytoplasmic PCR-RFLP approach. Tree Genetics & Genomes 6, 13–23 (2010). https://doi.org/10.1007/s11295-009-0224-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-009-0224-y