Abstract
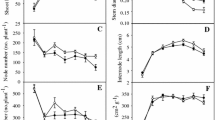
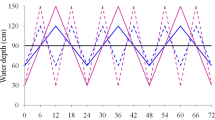
Although Spartina anglica C.E. Hubbard continues to be invasive in many countries, this species has experienced a drastic decline in coastal China over the last decade. We hypothesize that changes in the duration of tidal immersion were responsible for this decline because the elevation of the S. anglica-dominated area in coastal China has increased greatly over the last decade. We examined the effects of the duration of simulated tidal immersion and plant material provenance on growth, asexual reproduction, biomass accumulation, and allocation (percent of above-ground biomass to total biomass) of S. anglica in a greenhouse experiment. The provenance of S. anglica did not significantly affect any traits measured except for height, stalk diameter, and leaf area. However, all traits were affected by the duration of immersion. Plants grown under 6 h of immersion were taller and had more leaves, more roots, and larger leaf area than those under 2, 4, 8, and 10 h of immersion. Asexual traits and biomass of the plants grown under 6 h of immersion were significantly larger than those under other immersion durations. The results suggested that S. anglica benefits from tidal immersion and decreasing duration of tidal immersion may have resulted in the decline of the S. anglica populations in coastal China. Thus, controlling the duration of tidal immersion may be an effective way of controlling invasiveness of this species elsewhere in the world.




Similar content being viewed by others
References
Adams JB, Bate GC (1995) Ecological implications of tolerance of salinity and inundation by Spartina maritima. Aquat Bot 52:183–191
An SQ, Gu BH, Zhou CF, Wang ZS, Deng ZF, Zhi YB, Li HL, Chen L (2007) Spartina invasion in China: implications for invasive species management and future research. Weed Res 47:183–191
Armstrong W, Wright EJ, Lythe S, Gaynard TJ (1985) Plant zonation and the effects of the spring-neap tidal cycle on soil aeration in a Humber salt marsh. J Ecol 73:323–339
Ayres DR, Strong DR (2001) Origin and genetic diversity of Spartina anglica (Poaceae) using nuclear DNA markers. Am J Bot 88:1863–1867
Ayres DR, Debra LS, Katy Z, Shannon K, Donald RS (2004) Spread of exotic cordgrasses and hybrids (Spartina sp.) in the tidal marshes of San Francisco Bay, California, USA. Biol Invasions 6:221–231
Boorman LA, Hazelden J, Boorman M (2001) The effect of rates of sedimentation and tidal submersion regimes on the growth of salt marsh plants. Cont Shelf Res 21:2155–2165
Bradley PM, Morris JT (1990) Influence of oxygen and sulfide concentration on nitrogen uptake kinetics in Spartina alterniflora. Ecology 71:282–287
Brewer JS, Grace JB (1990) Plant community structure in an oligohaline tidal marsh. Vegetatio 90:93–107
Bridgewater P, Cresswell ID (1999) Biogeography of mangrove and salt marsh vegetation: implications for conservation and management in Australia. Mangrove Salt marsh 3:117–125
Browna CE, Pezeshki SR, DeLaune RD (2006) The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environ Exp Bot 58:140–148
Carlson PRJ, Forrest J (1982) Uptake of dissolved sulphide by Spartina alterniflora: evidence from natural sulphur isotope abundance ratios. Science 216:633–635
Chen LZ, Wang WQ, Lin P (2005) Photosynthetic and physiological responses of Kandelia candel L. Druce seedlings to duration of tidal immersion in artificial seawater. Environ Exp Bot 54:256–266
Chung CH (1985) Brief history of S. anglica and research work abroad. J Nanjing Univ (Special issue for research advances in Spartina, Natural Science) pp 1–30
Chung CH (1993) Thirty years of ecological engineering with Spartina plantations in China. Ecol Eng 2:261–289
Chung CH, Zhuo RZ, Xu GW (2004) Creation of Spartina plantations for reclaiming Dongtai, China, tidal flats and offshore sands. Ecol Eng 23:135–150
Colmer TD, Vos H, Pedersen O (2009) Tolerance of combined submergence and salinity in the halophytic stem-succulent Tecticornia pergranulata. Ann Bot 103:303–312
Cornick J, Standwerth A, Fisher PJ (2005) A preliminary study of fungal endophyte diversity in a stable and declining bed of Spartina anglica Hubbard. Mycologist 19:24–29
Daehler CC, Strong DR (1996) Status, prediction and prevention of introduced cordgrass Spartina spp. invasions in Pacific estuaries, USA. Biol Conserv 78:51–58
Dale MP, Causton DR (1992) The ecophysiology of Veronica chamaedrys, V. Montana and V. officinalis. II. The interaction of irradiance and water regime. J Ecol 80:493–504
de Souza MP, Yoch DC (1997) Spartina alterniflora dieback recovery correlates with increased acetylene reduction activity in salt marsh sediments. Estuar Coast Shelf Sci 45:547–555
Drew MC (1983) Plant injury and adaptation to oxygen deficiency in the root environment. Plant Soil 75:179–200
Drew MC, Lynch JM (1980) Soil anaerobiosis, micro-organisms and root function. Ann Rev Phytopathol 18:37–66
Erneberg M (1999) Effects of herbivores and competition on an introduced plant in decline. Oecologia 118:203–209
Etherington JR (1984) Comparative studies of plant growth and distribution in relation to waterlogging: X. Differential formation of adventitious roots and their experimental excision in Epilobium Hirsutum and Chamerion Angustifolium. J Ecol 72:389–404
Etherington R, Thomas M (1986) Responses to waterlogging and differential sensitivity to divalent iron and manganese in clones of Dactylisglomerata L. derived from well-drained and poorly drained soils. Ann Bot 58:109–119
Fagerstedt V, Crawford RM (1987) Is anoxia tolerance related to waterlogging tolerance? Funct Ecol 1:49–55
Fitter AH, Hay RKM (2002) Environmental physiology of plants, 3rd edn. Academic Press, San Diego
Fragoso G, Spencer T (2008) Physiographic control on the development of Spartina marshes. Science 322:1064
Gray AJ, Marshall DF, Raybould AF (1991) A century of evolution in Spartina anglica. Adv Ecol Res 21:1–62
Hacker SD, Bertness MD (1999) Experimental evidence for factors maintaining plant species diversity in a New England salt marsh. Ecology 80:2064–2073
Hacker SD, Heimer D, Hellquist CE, Reeder TG, Reeves B, Riordan TJ, Dethier MN (2001) A marine plant (Spartina anglica) invades widely varying habitats: potential mechanisms of invasion and control. Biol Invasions 3:211–217
Heathcote A, Daviesm S, Etheringtojn R (1987) Phenotypic flexibility of Carex flacca Schreb. Tolerance of soil flooding by populations from contrasting habitats. New Phytol 105:381–392
Howarth RW (1979) Pyrite: its rapid formation in a salt marsh and its importance to ecosystem metabolism. Science 203:49–51
Jackson MB, Armstrong W (1999) Formation of aerenchyma and the process of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Koch MS, Mendelssohn IA, McKee KL (1990) Mechanisms for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limno Oceanogr 35:399–408
Laan P, Tosserams M, Blom CWPM, Veen BW (1990) Internal oxygen transport in Rumex species and its significance for respiration under hypoxic condition. Plant Soil 122:39–46
Lenssern JPM, Menting FBJ, der Puttern WHV (2002) Plant response to simultaneous stress of waterlogging and shade: amplified or hierarchical effects? New Phytol 157:290–291
Lentz KA, Dunson WA (1998) Water level affects growth of endangered northeastern bulrush, Scirpus ancistrochaetus Schuyler. Aquat Bot 60:213–219
Li HL, An SQ, Zhi YB, Yan C, Zhao L, Zhou CF, Deng ZF, Su W, Liu YH (2008) Protogynous, pollen limitation and low seed production reasoned for the dieback of Spartina anglica in coastal China. Plant Sci 174:209–309
Li HL, Zhi YB, Lei GC, Zhao L, An SQ, Deng ZF (2010) Physiological responses of clonal plant Spartina anglica to simulated tidal waterlogging time. Wetland Sci 8(2):1–7
Maricle BR, Lee RW (2002) Aerenchyma development and oxygen transport in the estuarine cordgrasses Spartina alterniflora and S. anglica. Aquat Bot 74:109–120
McKee KL, Mendelssohn IA, Materne MD (2004) Acute salt marsh dieback in the Mississippi River Deltaic Plain: a drought-induced phenomenon? Global Ecol Biogeogr 13:67–73
Mendelssohn IA, McKee KL (1988) Spartina alterniflora die-back in Louisiana: time-course investigations of soil waterlogging effects. J Ecol 76:509–521
Mendoza R, Escudero V, Garcia I (2005) Plant growth, nutrient acquisition and mycorrhizal symbioses of a waterlogging tolerant legume (Lotus glaber Mill.) in a saline-sodic soil. Plant Soil 275:305–315
Partridge TR (1987) Spartina in New Zealand. New Zeal J Bot 25:567–575
Rengel Z (1992) The role of calcium in salt toxicity. Plant Cell Environ 15:625–632
Reynolds CE, Houle G, Marquis C (2000) Light and salinity affect growth of the salt marsh plant Aster Laurentianus. New Phytol 149:441–448
Roberts PD, Pullin AS (2007) The effectiveness of management interventions for the control of Spartina species: a systematic review and meta-analysis. Aquatic Conserv 18(5):592–618
Rubio G, Lavado RS (1999) Acquisition and allocation of resources in two waterlogging-tolerant grasses. New Phytol 143:539–546
Rybicki NB, Jenter HL, Carter V, Baltzer RA, Turtora M (1997) Observations of tidal flux between a submersed aquatic plant stand and the adjacent channel in the Potomac River near Washington, DC. Limnol Oceanogr 42:307–317
Sanchez JM, SanLeon DG, Izco J (2001) Primary colonisation of mudflat estuaries by Spartina maritima (Curtis) fernald in northwest Spain: vegetation structure and sediment accretion. Aquat Bot 69:15–25
Sanderson EW, Ustin SL, Foin TC (2000) The influence of tidal channels on the distribution of salt marsh plant species in Petaluma Marsh, CA, USA. Plant Ecol 146:29–41
Shiono K, Takahashi H, Colmer TD, Nakazono M (2008) Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci 175:52–58
Silvestri S, Defina A, Marani M (2005) Tidal regime, salinity and salt marsh plant zonation. Estuar Coast Shelf Sci 62:119–130
Thompson JD (1991) The biology of an invasive plant: what makes Spartina anglica so successful? Bioscience 4:393–401
Touchette BW (2006) Salt tolerance in a Juncus roemerianus brackish marsh: Spatial variations in plant water relations. J Exp Mar Biol Ecol 337:1–12
Van Hinsberg AJ, Van Tienderen PH (1997) Variation in growth form in relation to spectral light quality (red/far-ratio) in Plantago lanceolata L. in sun and shade populations. Oecologia 111:452–459
Visser EJW, Bogemann H, Van De Steeg GM, Pierik R, Blom CWPM (2000) Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol 148:93–103
Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM (2006) How plants cope with complete submergence. New Phytol 170:213–226
Waldren S, Davies MS, Etherington JR (1987) Comparative studies of plant growth and distribution in relation to waterlogging. XI. Growth of Geum rivale L. and Geum urbanum L. and soil chemical condition in experimentally flooded soil. New Phytol 105:551–562
Waldren S, Etherington JR, Davies MS (1988) Comparative studies of plant growth and distribution in relation to waterlogging. XV. The effect of waterlogging on growth of various populations of and hybrids between Geum rivale L. and Geum urbanum L. New Phytol 109:97–106
Wang WQ, Xiao Y, Chen LZ, Lin P (2007) Leaf anatomical responses to periodical waterlogging in simulated semidiurnal tides in mangrove Bruguiera gymnorrhiza seedlings. Aquat Bot 86:223–228
Wang N, Yu FH, Li PX, He WM, Liu J, Yu GL, Song YB, Dong M (2009) Clonal integration supports the expansion from terrestrial to aquatic environments of the amphibious stoloniferous herb Alternanthera philoxeroides. Plant Biol 11:483–489
Webb EC, Mendelssohn IA, Wilsey BJ (1995) Causes for vegetation dieback in a Louisiana salt marsh: a bioassay approach. Aquat Bot 51:281–289
Ye Y, Tam NFY, Wong YS, Lu CY (2003) Growth and physiological responses of two mangrove species (Bruguiera gymnorrhiza and Kandelia candel) to waterlogging. Environ Exp Bot 49:209–221
Yu FH, Wang N, Alpert P, He WM, Dong M (2009) Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. Am J Bot 96:1983–1989
Zhi YB, Li HL, An SQ, Zhao L, Zhou CF, Deng ZF (2007) Inter-specific competition: Spartina alterniflora is replacing Spartina anglica in coastal China. Estuar Coast Shelf Sci 74:437–448
Acknowledgments
We thank Guoyong Sun, Shicheng Lü, Jinjin Du, and Hui Wang for help with the field investigation, and Dr. Fei-Hai Yu and two anonymous reviewers for helpful comments. This research was supported by the Fundamental Research Funds for the Central Universities (YX2010-3), the Forestry Commonwealth Research Special Foundation of State Forestry Administration (200804005) and Jiangsu Natural Science Foundation (BK2007152).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, H., Lei, G., Zhi, Y. et al. Phenotypic responses of Spartina anglica to duration of tidal immersion. Ecol Res 26, 395–402 (2011). https://doi.org/10.1007/s11284-010-0794-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-010-0794-z