Abstract
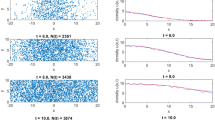
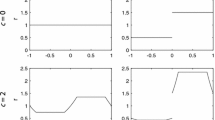
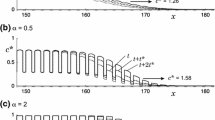
We investigate the speed of invasion waves for a single species generated by stochastic short- and/or long-distance colonizations in a time-continuous cellular automaton (CA) model on a two-dimensional homogenous landscape. By simulating the CA models, we demonstrate that stochasticity can dramatically increase the speed of invasion compared to the corresponding deterministic CA model or the corresponding one-dimensional stochastic CA model. To explain this phenomenon, we first develop a mathematical model for the invasion involving only short-distance colonization (i.e., colonization only occurs from the eight adjacent cells), and present several approximation methods for solving the model. Our analyses show that the increased wave speed in the stochastic model is due to irregularity in the shape of the wavefront. Further extension of this model to include long-distance colonization demonstrates that stochasticity influences speeds to even greater extents in this case. Using dimension analysis, we deduced a semi-empirical formula for the speed as a function of three parameters intrinsic to short- and long-distance colonization, which agrees well with simulation results. Based on these results, we discuss how important stochasticity in colonization and spatial dimensionality are in the acceleration of invasion speed.












Similar content being viewed by others
References
Andow D, Kareiva P, Levin S, Okubo A (1990) Spread of invading organisms. Landscape Ecol 4:177–188
Andow D, Kareiva P, Levin S, Okubo A (1993) Spread of invading organisms: patterns of spread. In: Kim KC (ed) Evolution of insect pests: the pattern of variations. Wiley, New York, pp 219–242
Broadbent SR, Kendall DG (1953) The random walk of Trichostrongylus retortaeformis. Biometrics 9:460–466
Buttel L, Durrett R, Levin S (2002) Competition and species packing in patchy environments. Theor Popul Biol 61:265–276
Cannas AS, Marco DE, Paez SA (2003) Modelling biological invasions: species traits, species interactions, and habitat heterogeneity. Math Biosci 183:93–110
Caswell H, Cohen JE (1991) Disturbance, interspecific interaction and diversity in metapopulations. Biol J Linn Soc 42:193–218
Caswell H, Etter RJ (1992) Ecological interactions in patchy environments: from patch-occupancy models to cellular automata. In: Levin SA, Powell TM, Steele JH (eds) Patch dynamics. Springer, Berlin Heidelberg New York, pp 93–l09
Caswell H, Etter RJ (1999) Cellular automaton models for competition in patchy environments: facilitation, inhibition, and tolerance. Bull Math Biol 61:625–649
Chave J, Muller-Landau HC, Levin SA (2002) Comparing classical community models: theoretical consequences for patterns of diversity. Am Nat 159:1–23
Clark JS (1998) Why trees migrate so fast: confronting theory with dispersal biology and the paleorecord. Am Nat 152:204–224
Clark JS, Lewis M, Horvath L (2001) Invasion by extremes: population spread with variation in dispersal and reproduction. Am Nat 157:537–554
Durrett R (1988) Lectures on particle systems and percolation. Wadsworth & Brooks/Cole, Pacific Grove, CA
Durrett R, Levin S (1994a) Stochastic spatial models: a user’s guide to ecological applications. Philos Trans R Soc Lond B 343:329–350
Durrett R, Levin SA (1994b) The importance of being discrete (and spatial). Theor Popul Biol 46:363–394
Durrett R, Levin S (1998) Spatial aspects of interspecific competition. Theor Popul Biol 53:30–43
Eden M (1961) A two dimensional growth process. In: Neyman J (ed) Proceedings of Fourth Berkeley Symposium on Math, Statistics and Probability. University of California Press, Berkeley, CA, pp 223–239
Ellner SP, Sasaki A, Haraguchi Y, Matsuda H (1998) Speed of invasion in lattice population models: pair-edge approximation. J Math Biol 36:469–484
Etter RJ, Caswell H (1994) The advantages of dispersal in a patchy environment: effects of disturbance in a cellular automaton model. In: Eckelbarger KJ, Young CM (eds) Reproduction, larval biology and recruitment in the deep-sea benthos. Columbia University Press, New York, pp 285–305
Filipe JAN, Maule MM (2004) Effects of dispersal mechanisms on spatio-temporal development of epidemics. J Theor Biol 226:125–141
Fisher RA (1937) The wave of advance of advantageous genes. Ann Eugen (Lond) 7:255–369
Harada Y, Iwasa Y (1994) Lattice population dynamics for plants with dispersing seeds and vegetative propagation. Res Popul Ecol 36:237–249
Harada Y, Ezoe H, Iwasa Y, Matsuda H, Sato K (1995) Population persistence and spatially limited social interaction. Theor Popul Biol 48:65–91
Hassell MP, Comins HN, May RM (1991) Spatial structure and chaos in insect population dynamics. Nature 353:255–258
Hengeveld R (1989) Dynamics of biology invasions. Chapman & Hall, London
Higgins SI, Nathan R, Cain ML (2003) Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology 84:1945–1956
Kot M, Lewis MA, van den Driessche P (1996) Dispersal data and the spread of invading organisms. Ecology 77:2027–2042
Kot M, Medlock J, Reluga T, Walton DB (2004) Stochasticity, invasions, and branching random walks. Theor Popul Biol 66:175–184
Kubo T, Iwasa Y, Furumoto N (1996) Forest spatial dynamics with gap expansion: total gap area and gap size distribution. J Theor Biol 180:229–246
Levin S (1992) The problem of pattern and scale in ecology: the Robert H MacArthur Award Lecture. Ecology 73:1943–1967
Levin SA, Durrett R (1997) From individuals to epidemics. Philos Trans R Soc Lond B 351:1615–1621
Lewis MA (2000) Spread rate for a nonlinear stochastic invasion. J Math Biol 41:430–454
Lewis MA, Pacala S (2000) Modeling and analysis of stochastic invasion processes. J Math Biol 41:387–429
Metz JAJ, Mollison D, van den Bosch F (2000) The dynamics of invasion waves. In: Dieckmann U, Law R, Metz JAJ (eds) The geometry of ecological interactions simplifying spatial complexity. Cambridge University Press, Cambridge, pp 482–512
Mollison D (1977) Spatial contact model for ecological and epidemic spread. J R Stat Soc 39:283–326
Mollison D, Kuulasmaa K (1985) Spatial epidemic models: theory and simulations. In: Bacon PJ (ed) Population dynamics of rabies in wildlife. Academic, London, pp 291–309
Nakamaru M, Levin SA (2004) Spread of two linked social norms on complex interaction networks. J Theor Biol 230:57–62
Nakamaru M, Matsuda H, Iwasa Y (1996) The evolution of cooperation in a lattice-structured population. J Theor Biol 184:65–81
Neubert MG, Caswell H (2000) Demography and dispersal: calculation and sensitivity analysis of invasion speed for structured populations. Ecology 81:1613–1628
Nowak MA, May RM, Sigmund K (1995) The arithmetics of mutual help. Sci Am 272(6):76–81
Okubo A (1980) Diffusion and ecological problems: mathematical models. Springer, Berlin Heidelberg New York
Okubo A, Levin S (2001) Diffusion and ecological problems: new perspectives, 2nd edn. Springer, Berlin Heidelberg New York
Petrovskii SV, Malchow H, Hilker FM, Venturino E (2005) Patterns of patchy spread in deterministic and stochastic models of biological invasion and biological control. Biol Invasions 7:771–793
Richardson D (1973) Random growth in a tessellation. Proc Camb Philos Soc 74:515–528
Satake A, Iwasa Y, Hakoyama H, Hubbell SP (2004) Estimating local interaction from spatiotemporal forest data, and Monte Carlo bias correction. J Theor Biol 226:225–235
Sato K, Matsuda H, Sasaki A (1994) Pathogen invasion and host extinction in lattice structured populations. J Math Biol 32:251–268
Shaw MW (1994) Modeling stochastic processes in plant pathology. Annu Rev Phytopathol 32:523–544
Shigesada N (1980) Spatial distribution of dispersing animals. J Math Biol 9:85–96
Shigesada N, Kawasaki K (1997) Biological invasions: theory and practice. Oxford University Press, Oxford
Shigesada N, Kawasaki K (2002) Invasion and species range expansion: effects of long-distance dispersal. In: Bullock J, Kenward R, Hails R (eds) Dispersal ecology. Blackwell, Oxford, pp 350–373
Shigesada N, Kawasaki K, Takeda Y (1995) Modeling stratified diffusion in biological invasions. Am Nat 146:229–251
Skellam JG (1951) Random dispersal in theoretical populations. Biometrika 38:196–218
Snyder RE (2003) How demographic stochasticity can slow biological invasions. Ecology 84:1333–1339
Tainaka K (1988) Lattice model for the Lotka–Volterra system. J Phys Soc Jpn 57:2588–2590
Tilman D, Lehman LL, Yin C (1997) Habitat destruction, dispersal, and deterministic extinction in competitive communities. Am Nat 149:407–435
Van den Bosch F, Hengeveld R, Metz JAJ (1992) Analyzing the velocity of animal range expansion. J Biogeogr 19:135–150
Williamson M (1996) Biological invasions. Chapman & Hall, London
Acknowledgments
We thank N. Osawa, K. Uehara, T. Takayanagi, C. Mukaino, and S. Baba for their contribution at the initial stage of this study. We also appreciate Dr S. Takahashi for valuable comments. Part of this study was supported by the Grant-in-Aid for Scientific Research Fund from the Japan Ministry of Education, Science, Culture and Sports (no. 13640627 and no. 09NP1501). HC acknowledges support from NSF Grant DEB-0235692.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kawasaki, K., Takasu, F., Caswell, H. et al. How does stochasticity in colonization accelerate the speed of invasion in a cellular automaton model?. Ecol Res 21, 334–345 (2006). https://doi.org/10.1007/s11284-006-0166-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-006-0166-x