Abstract
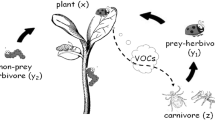
In response to feeding by phytophagous arthropods, plants emit volatile chemicals. This is shown to be an active physiological response of the plant and the released chemicals are therefore called herbivore-induced plant volatiles (HIPV). One of the supposed functions of HIPV for the plant is to attract carnivorous natural enemies of herbivores. Depending on which plant and herbivore species interact, blends of HIPV show qualitative and quantitative variation. Hence, one may ask whether this allows the natural enemies to discriminate between volatiles from plants infested by herbivore species that are either suitable or unsuitable as a food source for the natural enemy. Another question is whether natural enemies can also recognise HIPV when two or more herbivore species that differ in suitability as a food source simultaneously attack the same plant species. By reviewing the literature we show that arthropod predators and parasitoids can tell different HIPV blends apart in several cases of single plant–single herbivore systems and even in single plant–multiple herbivore systems. Yet, there are also cases where predators and parasitoids do not discriminate or discriminate only after having learned the association between HIPV and herbivores that are either suitable or non-suitable as a source of food. In this case, suitable herbivores may profit from colonising plants that are already infested by another non-suitable herbivore. The resulting temporal or partial refuge may have important population dynamical consequences, as such refuges have been shown to stabilise otherwise unstable predator–prey models of the Lotka-Volterra or Nicholson-Bailey type.
Similar content being viewed by others
References
Agrawal AA, Colfer RG (2000) Consequences of thrips-infested plants for attraction of conspecifics and parasitoids. Ecol Entomol 25:493–496
Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949
Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH (2000) Identification and synthesis of volicitin and related components from beet armyworm oral secretions. J Chem Ecol 26:203–220
Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135:1–13
Birkett MA, Chamberlain K, Guerrieri E, Pickett JA, Wadhams LJ, Yasuda T (2003) Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J Chem Ecol 29:1589–1600
Bouwmeester HJ, Verstappen FWA, Posthumus MA, Dicke M (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol 121:173–180
De Boer JG, Dicke M (2004) Experience with methyl salicylate affects behavioural responses of a predatory mite to blends of herbivore-induced plant volatiles. Entomol Exp App 110:181–189
De Boer JG, Posthumus MA, Dicke M (2004) Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol 30:2215–2230
De Boer JG, Snoeren TAL, Dicke M (2005) Predatory mites learn to discriminate between plant volatiles induced by prey and nonprey herbivores. Anim Behav 69:869–879
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
Dicke M (1994) Local and systemic production of volatile herbivore-induced terpenoids—their role in plant-carnivore mutualism. J Plant Physiol 143:465–472
Dicke M (1999) Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp App 91:131–142
Dicke M, Sabelis MW (1988) How plants obtain predatory mites as bodyguards. Neth J Zool 38:148–165
Dicke M, van Beek TA, Posthumus MA, Ben Dom N, van Bokhoven H, de Groot Æ (1990a) Isolation and identification of volatile kairomone that affects acarine predator–prey interactions: involvement of host plant in its production. J Chem Ecol:381–396
Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990b) Plant strategies of manipulating predator–prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol 16:3091–3118
Dicke M, de Boer JG, Hofte M, Rocha-Granados MC (2003) Mixed blends of herbivore-induced plant volatiles and foraging success of carnivorous arthropods. Oikos 101:38–48
Drukker B, Bruin J, Jacobs G, Kroon A, Sabelis MW (2000a) How predatory mites learn to cope with variability in volatile plant signals in the environment of their herbivorous prey. Exp Appl Acarol 24:881–895
Drukker B, Bruin J, Sabelis MW (2000b) Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiol Entomol 25:260–265
Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368
Geervliet JBF, Ariens S, Dicke M, Vet LEM (1998a) Long-distance assessment of patch profitability through volatile infochemicals by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae). Biol Control 11:113–121
Geervliet JBF, Vreugdenhil AI, Dicke M, Vet LEM (1998b) Learning to discriminate between infochemicals from different plant-host complexes by the parasitoids Cotesia glomerata and C. rubecula. Entomol Exp Appl 86:241–252
Gols R, Roosjen M, Dijkman H, Dicke M (2003) Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J Chem Ecol 29:2651–2666
Gu H, Dorn S (2000) Genetic variation in behavioral response to herbivore-infested plants in the parasitic wasp Cotesia glomerata (L.) (Hymenoptera: Braconidae). J Insect Behav 13:141–156
Guerrieri E, Poppy GM, Powell W, Tremblay E, Pennacchio F (1999) Induction and systemic release of herbivore-induced plant volatiles mediating in-flight orientation of Aphidius ervi. J Chem Ecol 25:1247–1261
Hassell MP (1978) The dynamics of arthropod predator-prey systems. Monographs in population biology 13. Princeton University Press, Princeton, 237 pp
Hopke J, Donath J, Blechert S, Boland W (1994) Herbivore-induced volatiles—the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a beta-glucosidase and jasmonic acid. FEBS Lett 352:146–150
Jia F, Margolies DC, Boyer JE, Charlton RE (2002) Genetic variation in foraging traits among inbred lines of a predatory mite. Heredity 89:371–379
Kant MR, Ament K, Sabelis MW, Haring M, Schuurink R (2004) Differential timing of spider mite-induced direct and indirect-defenses in tomato plants. Plant Physiol 135:483–495
Krips OE, Willems PEL, Gols R, Posthumus MA, Dicke M (1999) The response of Phytoseiulus persimilis to spider mite-induced volatiles from Gerbera: influence of starvation and experience. J Chem Ecol 25:2623–2641
Maeda T, Takabayashi J, Yano S, Takafuji A (1999) Response of the predatory mite, Amblyseius womersleyi (Acari: Phytoseiidae), toward herbivore-induced plant volatiles: variation in response between two local populations. Appl Entomol Zool (Jpn) 34:449–454
Maeda T, Takabayashi J, Yano S, Takafuji A (2001) Variation in the olfactory response of predatory mite, Amblyseius womersleyi (Acari: Phytoseiidae), of 13 populations to herbivore-induced plant volatiles. Exp Appl Acarol 25:55–64
Margolies DC, Sabelis MW, Boyer JE (1997) Response of a phytoseiid predator to herbivore-induced plant volatiles: selection on attraction and effect of prey exploitation. J Insect Behav 10:695–709
Mattiacci L, Dicke M, Posthumus MA (1995) β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts hostsearching parasitic wasps. Proc Natl Acad Sci USA 92:2036–2040
Mercke P, Kappers IF, Verstappen FWA, Vorst O, Dicke M, Bouwmeester HJ (2004) Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiol 135:2012–2024
Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathway for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41:391–398
Papaj DR, Lewis AC (1993) Insect learning: ecology and evolutionary perspectives. Chapman and Hall, NY
Papaj DR, Snellen H, Swaans K, Vet LEM (1994) Unrewarding experiences and their effect on foraging in the parasitic wasp Leptopilina heterotoma (Hymenoptera, Eucoilidae). J Insect Behav 7:465–481
Paré PW, Lewis WJ, Tumlinson JH (1999) Induced plant volatiles: biochemistry and effects on parasitoids. In: Agrawal AA, Tuzun S, Bent E (eds) Induced defenses against pathogens and herbivores. APS, St. Paul, MN, pp 167–180
Reymond P, Bodenhausen N, van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE (2004) A conserved transcriptional pattern in response to a specialist and a generalist herbivore. Plant Cell 16:3132–3147
Roitberg B, Reid ML, Li C (1993) Choosing hosts and mates: the value of learning. In: Papaj DR, Lewis AC (eds) Insect learning: ecology and evolutionary perspectives. Chapman and Hall, NY, pp 174–194
Sabelis MW, Bakker FM (1992) How predatory mites cope with the web of their tetranychid prey—a functional view on dorsal chaetotaxy in the Phytoseiidae. Exp Appl Acarol 16:203–225
Sabelis MW, van de Baan HE (1983) Location of distant spider mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol Exp Appl 33:303–314
Sabelis MW, Afman BP, Slim PJ (1984) Location of distant spider-mite colonies by Phytoseiulus persimilis: localization and extraction of a kairomone. In: Griffiths DA, Bowman CE (eds) Acarology VI, vol 1. Halsted, NY, pp 431–440
Sabelis MW, Diekmann O, Jansen VAA (1991) Metapopulation persistence despite local extinction: predator-prey patch models of the Lotka-Volterra type. Biol J Linn Soc 42:267–283
Sabelis MW, Janssen A, Pallini A, Venzon M, Bruin J, Drukker B, Scutareanu P (1999a) Behavioural responses of predatory and herbivorous arthropods to induced plant volatiles: from evolutionary ecology to agricultural applications. In: Agrawal AA, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores. American Phytopathological Society, St. Paul, MN, pp 269–296
Sabelis MW, van Baalen M, Bakker FM, Bruin J, Drukker B, Egas M, Janssen A, Lesna I, Pels B, van Rijn PCJ, Scutareanu P (1999b) The evolution of direct and indirect plant defence against herbivorous arthropods. In: Olff H, Brown VK, Drent RH (eds) Herbivores: between plants and predators. Blackwell, Oxford, pp 109–166
Sabelis MW, Janssen A, Bruin J, Bakker FM, Drukker B, Scutareanu P, van Rijn PCJ (1999c) Interactions between arthropod predators and plants: a conspiracy against herbivorous arthropods? In: Bruin J, van der Geest LPS, Sabelis MW (eds) Ecology and evolution of the acari. Series entomologica, vol. 55. Kluwer Academic, Dordrecht, pp 207–230
Sabelis MW, van Baalen M, Pels B, Egas M, Janssen A (2002) Evolution of exploitation and defence in plant–herbivore–predator interactions. In: Dieckmann U, Metz JAJ, Sabelis MW, Sigmund K (eds) The adaptive dynamics of infectious diseases. In pursuit of virulence management. Cambridge University Press, Cambridge, UK, pp 297–321
Sabelis MW, Takabayashi J, Janssen A, Kant MR, van Wijk M, Sznajder B, Aratchige NS, Lesna I, Belliure B, Schuurink RC (2005a) Ecology meets plant physiology: herbivore-induced plant responses and their indirect effects on arthropod communities. In: Ohgushi T, Craig TP, Price PW (eds) Indirect interaction webs: nontrophic linkages through induced plant traits. Cambridge University Press, Cambridge
Sabelis MW, Janssen A, Diekmann O, Jansen VAA, van Gool E, van Baalen M (2005b) Global persistence despite local extinction in acarine predator-prey systems: lessons from experimental and mathematical exercises. Adv Ecol Res 37:183–220
Scutareanu P, Drukker B, Bruin J, Posthumus MA, Sabelis MW (1997) Isolation and identification of volatile synomones involved in the interaction between psylla-infested pear trees and two anthocorid predators. J Chem Ecol 23:2241–2260
Shimoda T, Dicke M (2000) Attraction of a predator to chemical information related to nonprey: when can it be adaptive? Behav Ecol 11:606–613
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2001) Infochemically mediated tritrophic interaction webs on cabbage plants. Popul Ecol 43:23–29
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2002) Oviposition preferences of herbivores are affected by tritrophic interaction webs. Ecol Lett 5:186–192
Storeck A, Poppy GM, van Emden HF, Powell W (2000) The role of plant chemical cues in determining host preference in the generalist aphid parasitoid Aphidius colemani. Entomol Exp Appl 97:41–46
Takabayashi J, Dicke M (1992) Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol Exp Appl 64:187–193
Takabayashi J, Dicke M (1996) Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci 1:109–113
Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2:1–6
Thaler JS, Farag MA, Paré PW, Dicke M (2002) Jasmonate-deficient plants have reduced direct and indirect defenses against herbivores. Ecol Lett 5:764–774
Turlings TCJ, Tumlinson JH (1992) Systemic release of chemical signals by herbivore-injured corn. Proc Natl Acad Sci USA 89:8399–8402
Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host seeking parasitic wasps. Science 250:1251–1253
Turlings TCJ, Wäckers F, Vet LEM, Lewis WJ, Tumlinson JH (1993) Learning of host-finding cues by hymenopterous parasitoids. In: Papaj DR, Lewis AC (eds) Insect learning: ecology and evolutionary perspectives. Chapman and Hall, NY, pp 51–78
Turlings TCJ, Loughrin JH, McCall PJ, Rose USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92:4169–4174
Van Poecke RMP, Dicke M (2003) Signal transduction downstream of salicylic and jasmonic acid in herbivory-induced parasitoid attraction by Arabidopsis is independent of JAR1 and NPR1. Plant Cell Environ 26:1541–1548
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Ecol Syst 37:141–172
Vet LEM, Lewis WJ, Cardé RT (1995) Parasitoid foraging and learning. In: Cardé RT, Bell WJ (eds) Chemical ecology of insects 2. Chapman and Hall, NY, pp 65–101
Vos M, Berrocal SM, Karamaouna F, Hemerik L, Vet LEM (2001) Plant-mediated indirect effects and the persistence of parasitoid-herbivore communities. Ecol Lett 4:38–45
Wang Q, Dorn S (2003) Selection on olfactory response to semiochemicals from a host-plant complex in a parasitic wasp. Heredity 91:430–435
Wang Q, Gu H, Dorn S (2004) Genetic relationship between olfactory response and fitness in the Cotesia glomerata (L.). Heredity 92:579–584
Acknowledgements
This study was supported by CREST of JST (Japan Science and Technology Corporation), and by the Grant for the Biodiversity Research of the 21st Century COE (A14).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takabayashi, J., Sabelis, M.W., Janssen, A. et al. Can plants betray the presence of multiple herbivore species to predators and parasitoids? The role of learning in phytochemical information networks. Ecol Res 21, 3–8 (2006). https://doi.org/10.1007/s11284-005-0129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-005-0129-7