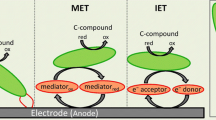
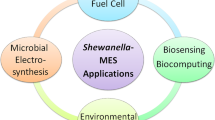
Abstract
Electrogens are very common in nature and becoming a contemporary theme for research as they can be exploited for extracellular electron transfer. Extracellular electron transfer is the key mechanism behind bioelectricity generation and bioremediation of pollutants via microbes. Extracellular electron transfer mechanisms for electrogens other than Shewanella and Geobacter are less explored. An efficient extracellular electron transfer system is crucial for the sustainable future of bioelectrochemical systems. At present, the poor extracellular electron transfer efficiency remains a decisive factor in limiting the development of efficient bioelectrochemical systems. In this review article, the EET mechanisms in different electrogens (bacteria and yeast) have been focused. Apart from the well-known electron transfer mechanisms of Shewanella oneidensis and Geobacter metallireducens, a brief introduction of the EET pathway in Rhodopseudomonas palustris TIE-1, Sideroxydans lithotrophicus ES-1, Thermincola potens JR, Lysinibacillus varians GY32, Carboxydothermus ferrireducens, Enterococcus faecalis and Saccharomyces cerevisiae have been included. In addition to this, the article discusses the several approaches to anode modification and genetic engineering that may be used in order to increase the rate of extracellular electron transfer. In the side lines, this review includes the engagement of the electrogens for different applications followed by the future perspective of efficient extracellular electron transfer.









Similar content being viewed by others
References
Aiyer KS (2020) How does electron transfer occur in microbial fuel cells? World J Microbiol Biotechnol 36:1–9
Altamura L, Horvath C, Rengaraj S et al (2017) A synthetic redox biofilm made from metalloprotein–prion domain chimera nanowires. Nat Chem 9:157–163
Angelaalincy MJ, Navanietha Krishnaraj R, Shakambari G et al (2018) Biofilm Engineering Approaches for improving the performance of Microbial Fuel cells and Bioelectrochemical Systems. Front Energy Res 6:1–12. https://doi.org/10.3389/fenrg.2018.00063
Arunasri K, Mohan SV (2019) Biofilms: microbial life on the electrode surface. Microbial Electrochemical Technology. Elsevier, pp 295–313
Atkinson JT, Chavez MS, Niman CM, El-Naggar MY (2022) Living electronics: a catalogue of engineered living electronic components. Microb Biotechnol 1–27. https://doi.org/10.1111/1751-7915.14171
Atkinson S, Williams P (2009) Quorum sensing and social networking in the microbial world. J R Soc Interface 6:959–978
Awate B, Steidl RJ, Hamlischer T, Reguera G (2017) Stimulation of electro-fermentation in single-chamber microbial electrolysis cells driven by genetically engineered anode biofilms. J Power Sources 356:510–518
Barbosa SG, Peixoto L, Alves JI, Alves MM (2021) Bioelectrochemical systems (BESs) towards conversion of carbon monoxide/syngas: a mini-review. Renew Sustain Energy Rev 135:110358
Baron D, LaBelle E, Coursolle D et al (2009) Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J Biol Chem 284:28865–28873
Bautista EM, Alexander M (1972) Reduction of inorganic compounds by soil microorganisms. Soil Sci Soc Am J 36:918–920
Belchik SM, Kennedy DW, Dohnalkova AC et al (2011) Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 77:4035–4041. https://doi.org/10.1128/AEM.02463-10
Bjerg JT, Boschker HTS, Larsen S et al (2018) Long-distance electron transport in individual, living cable bacteria. Proc Natl Acad Sci 115:5786–5791
Blumberger J (2018) Electron transfer and transport through multi-heme proteins: recent progress and future directions. Curr Opin Chem Biol 47:24–31
Boedicker JQ, Gangan M, Naughton K et al (2021) Engineering Biological Electron transfer and redox pathways for nanoparticle synthesis. Bioelectricity 3:126–135
Brookshaw DR, Coker VS, Lloyd JR et al (2014) Redox interactions between cr (VI) and Fe (II) in bioreduced biotite and chlorite. Environ Sci Technol 48:11337–11342
Byrne-Bailey KG, Wrighton KC, Melnyk RA et al (2010) Complete genome sequence of the electricity-producing “Thermincola potens” strain JR. J Bacteriol 192:4078–4079
Byrne JM, Coker VS, Moise S et al (2013) Controlled cobalt doping in biogenic magnetite nanoparticles. J R Soc Interface 10:20130134
Carlson HK, Iavarone AT, Gorur A et al (2012) Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria. Proc Natl Acad Sci U S A 109:1702–1707. https://doi.org/10.1073/pnas.1112905109
Chai L, Ding C, Li J et al (2019) Multi-omics response of Pannonibacter phragmitetus BB to hexavalent chromium. Environ Pollut 249:63–73
Chao L, Rakshe S, Leff M, Spormann AM (2013) PdeB, a cyclic di-GMP-specific phosphodiesterase that regulates Shewanella oneidensis MR-1 motility and biofilm formation. J Bacteriol 195:3827–3833
Chen L, Wu Y, Shen Q et al (2022) Enhancement of hexavalent chromium reduction by Shewanella oneidensis MR-1 in presence of copper nanoparticles via stimulating bacterial extracellular electron transfer and environmental adaptability. Bioresour Technol 361:127686. https://doi.org/10.1016/j.biortech.2022.127686
Cheng J, Xia R, Li H et al (2022) Enhancing Extracellular Electron transfer of Geobacter sulfurreducens in Bioelectrochemical Systems using N-Doped Fe3O4@Carbon dots. ACS Sustain Chem Eng 10:3935–3950. https://doi.org/10.1021/acssuschemeng.1c08167
Cheng Q, Call DF (2021) Developing microbial communities containing a high abundance of exoelectrogenic microorganisms using activated carbon granules. Sci Total Environ 768:144361
Cheng Z, Xiong J, Min D et al (2020) Promoting bidirectional extracellular electron transfer of Shewanella oneidensis MR-1 for hexavalent chromium reduction via elevating intracellular cAMP level. Biotechnol Bioeng 117:1294–1303
Chiranjeevi P, Patil SA (2020) Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies11Supplementary information (SI) available. Biotechnol Adv 39:107468. https://doi.org/10.1016/j.biotechadv.2019.107468
Choudhury P, Prasad Uday US, Bandyopadhyay TK et al (2017) Performance improvement of microbial fuel cell (MFC) using suitable electrode and bioengineered organisms: a review. Bioengineered 8:471–487
Costa NL, Clarke TA, Philipp LA et al (2018) Electron transfer process in microbial electrochemical technologies: the role of cell-surface exposed conductive proteins. Bioresour Technol 255:308–317. https://doi.org/10.1016/j.biortech.2018.01.133
Crean DE, Coker VS, Van Der Laan G, Lloyd JR (2012) Engineering biogenic magnetite for sustained cr (VI) remediation in flow-through systems. Environ Sci Technol 46:3352–3359
Cutting RS, Coker VS, Telling ND et al (2010) Optimizing cr (VI) and tc (VII) remediation through nanoscale biomineral engineering. Environ Sci Technol 44:2577–2584
Das T, Sharma PK, Busscher HJ et al (2010) Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl Environ Microbiol 76:3405–3408
Dikow RB (2011) Genome-level homology and phylogeny of Shewanella (Gammaproteobacteria: lteromonadales: Shewanellaceae). BMC Genomics 12:1–14
Dong G, Chen Y, Yan Z et al (2020) Recent advances in the roles of minerals for enhanced microbial extracellular electron transfer. https://doi.org/10.1016/j.rser.2020.110404
Elakkiya E, Niju S (2021) Bioelectrochemical treatment of real-field bagasse-based paper mill wastewater in dual-chambered microbial fuel cell. 3 Biotech 11:1–10
Emerson D, Moyer C (1997) Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol 63:4784–4792
Faustino MM, Fonseca BM, Costa NL et al (2021) Crossing the wall: characterization of the multiheme cytochromes involved in the extracellular electron transfer pathway of Thermincola ferriacetica. Microorganisms 9:293
Ferguson SJ, Ingledew WJ (2008) Energetic problems faced by micro-organisms growing or surviving on parsimonious energy sources and at acidic pH: I. Acidithiobacillus ferrooxidans as a paradigm. Biochim Biophys Acta (BBA)-Bioenergetics 1777:1471–1479
Fernandes AP, Nunes TC, Paquete CM, Salgueiro CA (2017) Interaction studies between periplasmic cytochromes provide insights into extracellular electron transfer pathways of Geobacter sulfurreducens. Biochem J 474:797–808
Finkenstadt VL (2005) Natural polysaccharides as electroactive polymers. Appl Microbiol Biotechnol 67:735–745
Fredrickson JK, Zachara JM, Kennedy DW et al (2004) Reduction of TcO4 – by sediment-associated biogenic Fe (II). Geochim Cosmochim Acta 68:3171–3187
Gao K, Lu Y (2021) Putative extracellular electron transfer in methanogenic archaea. Front Microbiol 12:649
Gavrilov SN, Zavarzina DG, Elizarov IM et al (2021) Novel extracellular Electron transfer channels in a gram-positive thermophilic bacterium. 11:1–21. https://doi.org/10.3389/fmicb.2020.597818
Ghani B, Takai M, Hisham NZ et al (1993) Isolation and characterization of a Mo6+-reducing bacterium. Appl Environ Microbiol 59:1176–1180
Ghasemi R, Fatemi F, Mir-Derikvand M, Zarei M (2020) Evaluation of mtr cluster expression in Shewanella RCRI7 during uranium removal. Arch Microbiol 202:2711–2726
Gomaa OM, Costa NL, Paquete CM (2022) Electron transfer in Gram-positive bacteria: enhancement strategies for bioelectrochemical applications. World J Microbiol Biotechnol 38. https://doi.org/10.1007/s11274-022-03255-y
Gong Y, Werth CJ, He Y et al (2018) Intracellular versus extracellular accumulation of Hexavalent chromium reduction products by Geobacter sulfurreducens PCA. Environ Pollut 240:485–492
Gonzalez R, Gentina JC, Acevedo F (2004) Biooxidation of a gold concentrate in a continuous stirred tank reactor: mathematical model and optimal configuration. Biochem Eng J 19:33–42
Gu Y, Qi X, Yang X et al (2023) Extracellular electron transfer and the conductivity in microbial aggregates during biochemical wastewater treatment: a bottom-up analysis of existing knowledge. Water Res 231:119630. https://doi.org/10.1016/j.watres.2023.119630
Guannan K, Yonggang Y, Yeshen L et al (2022) Cysteine-mediated Extracellular Electron transfer of Lysinibacillus varians GY32. Microbiol Spectr 10:e02798–e02722. https://doi.org/10.1128/spectrum.02798-22
Guerra-Santos L, Käppeli O, Fiechter A (1984) Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Environ Microbiol 48:301–305
Gupta D, Sutherland MC, Rengasamy K et al (2019) Photoferrotrophs produce a PioAB electron conduit for extracellular electron uptake. MBio 10:e02668–e02619
Guzman MS, Rengasamy K, Binkley MM et al (2019) Phototrophic extracellular electron uptake is linked to carbon dioxide fixation in the bacterium Rhodopseudomonas palustris. Nat Commun 10:1–13
Hädrich A, Taillefert M, Akob DM et al (2019) Microbial Fe (II) oxidation by Sideroxydans lithotrophicus ES-1 in the presence of Schlöppnerbrunnen fen-derived humic acids. FEMS Microbiol Ecol 95:fiz034
Hallbeck L, Sthl F, Pedersen K (1993) Phytogeny and phenotypic characterization of the stalk-forming and iron-oxidizing bacterium Gallionella ferruginea. Microbiology 139:1531–1535
Han S, Gao X, Ying H, Zhou CC (2016) NADH gene manipulation for advancing bioelectricity in Clostridium ljungdahlii microbial fuel cells. Green Chem 18:2473–2478
He S, Barco RA, Emerson D, Roden EE (2017) Comparative genomic analysis of neutrophilic iron (II) oxidizer genomes for candidate genes in extracellular electron transfer. Front Microbiol 8:1584
Hederstedt L, Gorton L, Pankratova G (2020) Two routes for extracellular electron transfer in Enterococcus faecalis. J Bacteriol 202:e00725–e00719
Holmes DE, Smith JA (2016) Biologically produced methane as a renewable energy source. Adv Appl Microbiol 97:1–61
Ing NL, El-Naggar MY, Hochbaum AI (2018) Going the Distance: long-range conductivity in protein and peptide Bioelectronic materials. J Phys Chem B 122:10403–10423. https://doi.org/10.1021/acs.jpcb.8b07431
Iram A, Cekmecelioglu D, Demirci A (2020) Ideal feedstock and fermentation process improvements for the production of lignocellulolytic enzymes. Processes 9:38
Iwahori K, Takeuchi F, Kamimura K, Sugio T (2000) Ferrous iron-dependent volatilization of mercury by the plasma membrane of Thiobacillus ferrooxidans. Appl Environ Microbiol 66:3823–3827
Jiang S, Hur H-G (2013) Effects of the anaerobic respiration of Shewanella oneidensis MR-1 on the stability of extracellular U (VI) nanofibers. Microbes Environ ME12149
Jiang Z, Shi M, Shi L (2020) Degradation of organic contaminants and steel corrosion by the dissimilatory metal-reducing microorganisms Shewanella and Geobacter spp. Int Biodeterior Biodegradation 147:104842
Jiménez Otero F, Chan CH, Bond DR (2018) Identification of different putative outer membrane electron conduits necessary for Fe (III) citrate, Fe (III) oxide, mn (IV) oxide, or electrode reduction by Geobacter sulfurreducens. J Bacteriol 200:e00347–e00318
Karygianni L, Ren Z, Koo H, Thurnheer T (2020) Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 28:668–681
Kashefi K, Tor JM, Nevin KP, Lovley DR (2001) Reductive precipitation of gold by dissimilatory Fe (III)-reducing bacteria and archaea. Appl Environ Microbiol 67:3275–3279
Kavanaugh JS, Flack CE, Lister J et al (2019) Identification of extracellular DNA-binding proteins in the biofilm matrix. MBio 10:e01137–e01119
Keogh D, Lam LN, Doyle LE et al (2018) Extracellular electron transfer powers Enterococcus faecalis biofilm metabolism. MBio 9:e00626–e00617
Kimber RL (2019) Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis. Appl Click Chem 14
Konishi Y, Ohno K, Saitoh N et al (2007a) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653
Konishi Y, Tsukiyama T, Tachimi T et al (2007b) Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim Acta 53:186–192
Konovalova EY, Stom DI, Zhdanova GO et al (2018) The microorganisms used for working in microbial fuel cells. https://doi.org/10.1063/1.5031979. AIP Conf Proc 1952:
Kouzuma A, Meng X-Y, Kimura N et al (2010) Disruption of the putative cell surface polysaccharide biosynthesis gene SO3177 in Shewanella oneidensis MR-1 enhances adhesion to electrodes and current generation in microbial fuel cells. Appl Environ Microbiol 76:4151–4157
Kouzuma A, Oba H, Tajima N et al (2014) Electrochemical selection and characterization of a high current-generating Shewanella oneidensis mutant with altered cell-surface morphology and biofilm-related gene expression. BMC Microbiol 14:1–11
Krasowska A, Sigler K (2014) How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol 4:112
Krishna KV, Swathi K, Hemalatha M, Mohan SV (2019) Chap. 1.6 - Bioelectrocatalyst in Microbial Electrochemical Systems and Extracellular Electron Transport. In: Mohan SV, Varjani S, Pandey ABT-MET (eds) Biomass, Biofuels and Biochemicals. Elsevier, pp 117–141
Leang C, Malvankar NS, Franks AE et al (2013) Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production. Energy Environ Sci 6:1901–1908
Lee J-H, Han J, Choi H, Hur H-G (2007a) Effects of temperature and dissolved oxygen on Se (IV) removal and Se (0) precipitation by Shewanella sp HN-41. Chemosphere 68:1898–1905
Lee JH, Kim MG, Yoo B, Fredrickson JK, Sadowsky MJ, Hur HG et al (2007b) 201 Biogenic formation of photoactive arsenic-sulfide nanotubes by Shewanella sp 202 strain HN-41. Proc Natl Acad Sci USA 104:20410–20415
Li Y, Li Y, Chen Y et al (2022) Coupling riboflavin de novo biosynthesis and cytochrome expression for improving extracellular electron transfer efficiency in Shewanella oneidensis. Biotechnol Bioeng 119:2806–2818. https://doi.org/10.1002/bit.28172
Lies DP, Hernandez ME, Kappler A et al (2005) Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol 71:4414–4426
Liu J, Wang Z, Belchik SM et al (2012) Identification and characterization of M to A: a decaheme c-type cytochrome of the neutrophilic fe(ll)-oxidizing bacterium Sideroxydans lithotrophicus ES-1. Front Microbiol 3:1–11. https://doi.org/10.3389/fmicb.2012.00037
Liu X, Tremblay P-L, Malvankar NS et al (2014a) A Geobacter sulfurreducens strain expressing Pseudomonas aeruginosa type IV pili localizes OmcS on pili but is deficient in Fe (III) oxide reduction and current production. Appl Environ Microbiol 80:1219–1224
Liu Y, Fredrickson JK, Zachara JM, Shi L (2015a) Direct involvement of ombB, omaB, and omcB genes in extracellular reduction of Fe(III) by Geobacter sulfurreducens PCA. Front Microbiol 6:1–8. https://doi.org/10.3389/fmicb.2015.01075
Liu Y, Fredrickson JK, Zachara JM, Shi L (2015b) Direct involvement of ombB, omaB, and omcB genes in extracellular reduction of Fe (III) by Geobacter sulfurreducens PCA. Front Microbiol 6:1075
Liu Y, Wang Z, Liu J et al (2014b) A trans-outer membrane porin-cytochrome protein complex for extracellular electron transfer by Geobacter sulfurreducens PCA. Environ Microbiol Rep 6:776–785. https://doi.org/10.1111/1758-2229.12204
Lloyd JR (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425
Lloyd JR, Sole VA, Van Praagh CVG, Lovley DR (2000) Direct and Fe (II)-mediated reduction of technetium by Fe (III)-reducing bacteria. Appl Environ Microbiol 66:3743–3749
Lopes LVS, Dragunski DC, Pawlicka A, Donoso JP (2003) Nuclear magnetic resonance and conductivity study of starch based polymer electrolytes. Electrochim Acta 48:2021–2027
Louro RO, Costa NL, Fernandes AP et al (2019) Exploring the Molecular Mechanisms of Extracellular Electron transfer for harnessing reducing power in METs: methodologies and approaches. Microbial Electrochemical Technology. Elsevier, pp 261–293
Lovley DR, Coates JD (1997) Bioremediation of metal contamination. Curr Opin Biotechnol 8:285–289
Lovley DR, Phillips EJP, Gorby YA, Landa ER (1991) Microbial reduction of uranium. Nature 350:413–416
Lovley DR, Phillips EJP, Lonergan DJ (1989) Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol 55:700–706
Marsili E, Baron DB, Shikhare ID et al (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci 105:3968–3973
Martinez CM, Alvarez LH (2018) Application of redox mediators in bioelectrochemical systems. Biotechnol Adv 36:1412–1423. https://doi.org/10.1016/j.biotechadv.2018.05.005
McCall AD, Pathirana RU, Prabhakar A et al (2019) Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ biofilms microbiomes 5:1–12
McFarlane IR, Lazzari-Dean JR, El-Naggar MY (2015) Field effect transistors based on semiconductive microbially synthesized chalcogenide nanofibers. Acta Biomater 13:364–373
Meyer TE, Cusanovich MA (2003) Discovery and characterization of electron transfer proteins in the photosynthetic bacteria. Photosynth Res 76:111–126
Meysman FJR (2018) Cable bacteria take a new breath using long-distance electricity. Trends Microbiol 26:411–422
Min D, Cheng L, Zhang F et al (2017) Enhancing extracellular electron transfer of Shewanella oneidensis MR-1 through coupling improved flavin synthesis and metal-reducing conduit for pollutant degradation. Environ Sci Technol 51:5082–5089
Moß C, Behrens A, Schröder U (2020) The limits of Three-Dimensionality: systematic Assessment of Effective Anode macrostructure dimensions for mixed-culture Electroactive Biofilms. Chemsuschem 13:582–589. https://doi.org/10.1002/cssc.201902923
Nosek D, Jachimowicz P, Cydzik-Kwiatkowska A (2020) Anode modification as an alternative approach to improve electricity generation in microbial fuel cells. Energies 13:1–22. https://doi.org/10.3390/en13246596
Okamoto A, Hashimoto K, Nealson KH, Nakamura R (2013) Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc Natl Acad Sci 110:7856–7861
Pearce CI, Pattrick RAD, Law N et al (2009) Investigating different mechanisms for biogenic selenite transformations: Geobacter sulfurreducens, Shewanella oneidensis and Veillonella atypica. Environ Technol 30:1313–1326
Pirbadian S, El-Naggar MY (2012) Multistep hopping and extracellular charge transfer in microbial redox chains. Phys Chem Chem Phys 14:13802–13808
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc London Ser b Contain Pap a Biol character 84:260–276
Prasad D, Arun S, Murugesan M et al (2007) Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cell. Biosens Bioelectron 22:2604–2610
Raghavulu SV, Goud RK, Sarma PN, Mohan SV (2011) Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: influence of redox condition and substrate load. Bioresour Technol 102:2751–2757. https://doi.org/10.1016/j.biortech.2010.11.048
Rawlings DE, Dew D, du Plessis C (2003) Biomineralization of metal-containing ores and concentrates. TRENDS Biotechnol 21:38–44
Reguera G, Nevin KP, Nicoll JS et al (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72:7345–7348
Rengasamy K, Ranaivoarisoa T, Singh R, Bose A (2018) An insoluble iron complex coated cathode enhances direct electron uptake by Rhodopseudomonas palustris TIE-1. Bioelectrochemistry 122:164–173. https://doi.org/10.1016/j.bioelechem.2018.03.015
Rosenbaum M, Angenent LT (2010) Genetically modified microorganisms for bioelectrochemical systems.Bioelectrochemical Syst From Extracell electron Transf to Biotechnol Appl101–117
Sánchez-Baracaldo P, Cardona T (2020) On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol 225:1440–1446
Saunders SH, Edmund CM, Yates MD et al (2020) Extracellular DNA promotes efficient extracellular electron transfer by pyocyanin in Pseudomonas aeruginosa biofilms. Cell 182:919–932
Schröder U (2009) FUEL CELLS – EXPLORATORY FUEL CELLS | Microbial Fuel cells. Garche JBT-E of EPS. Elsevier, Amsterdam, pp 206–216
Sepúlveda Cisternas I, Salazar JC, García-Angulo VA (2018) Overview on the bacterial iron-riboflavin metabolic axis. Front Microbiol 9:1478
Sevda S, Sharma S, Joshi C et al (2018) Biofilm formation and electron transfer in bioelectrochemical systems. Environ Technol Rev 7:220–234
Shi L, Rosso KM, Zachara JM, Fredrickson JK (2012) Mtr extracellular electron-transfer pathways in Fe (III)-reducing or fe (II)-oxidizing bacteria: a genomic perspective
Shih C, Museth AK, Abrahamsson M et al (2008) Tryptophan-accelerated electron flow through proteins. Sci (80-) 320:1760–1762
Singh VK, Singh AL, Singh R, Kumar A (2018) Iron oxidizing bacteria: insights on diversity, mechanism of iron oxidation and role in management of metal pollution. Environ Sustain 1:221–231
Singh R, Ranaivoarisoa TO, Gupta D et al (2020) Genetic redundancy in iron and manganese transport in the metabolically versatile bacterium Rhodopseudomonas palustris TIE-1. Appl Environ Microbiol 86:e01057–e01020
Singha TK, Gulati P, Mohanty A et al (2017) Efficient genetic approaches for improvement of plasmid based expression of recombinant protein in Escherichia coli: a review. Process Biochem 55:17–31
Staicu LC, Barton LL (2017) Bacterial metabolism of selenium—for survival or profit. Bioremediation of selenium contaminated wastewater. Springer, pp 1–31
Stolz JF, Oremland RS (1999) Bacterial respiration of arsenic and selenium. FEMS Microbiol Rev 23:615–627
Sturm G, Richter K, Doetsch A et al (2015) A dynamic periplasmic electron transfer network enables respiratory flexibility beyond a thermodynamic regulatory regime. ISME J 9:1802–1811
Sugio T, Wakabayashi M, Kanao T, Takeuchi F (2008) Isolation and characterization of Acidithiobacillus ferrooxidans strain D3-2 active in copper bioleaching from a copper mine in Chile. Biosci Biotechnol Biochem 72:998–1004
Summers ZM, Ueki T, Ismail W et al (2012) Laboratory evolution of Geobacter sulfurreducens for enhanced growth on lactate via a single-base-pair substitution in a transcriptional regulator. ISME J 6:975–983
Sun D, Chen J, Huang H et al (2016) The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int J Hydrogen Energy 41:16523–16528. https://doi.org/10.1016/j.ijhydene.2016.04.163
Tahrioui A, Duchesne R, Bouffartigues E et al (2019) Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. npj Biofilms Microbiomes 5:1–11
Tan Y, Adhikari RY, Malvankar NS et al (2017) Expressing the Geobacter metallireducens PilA in Geobacter sulfurreducens yields pili with exceptional conductivity. MBio 8:e02203–e02216
Tapia JM, Munoz JA, Gonzalez F et al (2009) Interrelation between cells and extracellular polymeric substances (EPS) from Acidiphilium 3.2 sup (5) on carbon surfaces. Advanced materials research. Trans Tech Publ, pp 287–290
Teixeira LR, Fernandes TM, Silva MA et al (2022) Characterization of a Novel cytochrome involved in Geobacter sulfurreducens’ Electron Harvesting Pathways. Chem – A Eur J n. https://doi.org/10.1002/chem.202202333. /a:e202202333
Thunnissen D, Guernsey C, Baker R, Miyake R (2004) Advanced space storable propellants for outer planet exploration. In: 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit. p 3488
Valdés J, Pedroso I, Quatrini R et al (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9:1–24
Verma M, Mishra V (2021) Recent trends in upgrading the performance of yeast as electrode biocatalyst in microbial fuel cells. Chemosphere 284:131383
Walker DJF, Martz E, Holmes DE et al (2019) The archaellum of Methanospirillum hungatei is electrically conductive. MBio 10:e00579–e00519
Wang S, Liu X, Liu H et al (2015a) The exopolysaccharide Psl–eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ Microbiol Rep 7:330–340. https://doi.org/10.1111/1758-2229.12252
Wang Z, Shi Z, Shi L et al (2015b) Effects of soluble flavin on heterogeneous electron transfer between surface-exposed bacterial cytochromes and iron oxides. Geochim Cosmochim Acta 163:299–310
Wang Y-Z, Shen Y, Gao L et al (2017) Improving the extracellular electron transfer of Shewanella oneidensis MR-1 for enhanced bioelectricity production from biomass hydrolysate. RSC Adv 7:30488–30494
Wang P, Dong F, Wang X et al (2018a) Effects of riboflavin and AQS as electron shuttles on U (vi) reduction and precipitation by Shewanella putrefaciens. RSC Adv 8:30692–30700
Wang Y, Feng Y, Li H et al (2018b) Reduction of vanadium (V) in a microbial fuel cell: V (IV) Migration and Electron transfer mechanism. Int J Electrochem Sci 13:11024–11037
Wang B, Jiang Z, Yu JC et al (2019) Enhanced CO2 reduction and valuable C2 + chemical production by a CdS-photosynthetic hybrid system. Nanoscale 11:9296–9301. https://doi.org/10.1039/c9nr02896j
Wang Y, You L-X, Zhong H-L et al (2022a) Au(III)-induced extracellular electron transfer by Burkholderia contaminans ZCC for the bio-recovery of gold nanoparticles. Environ Res 210:112910. https://doi.org/10.1016/j.envres.2022.112910
Wang Z, Hu Y, Dong Y et al (2022b) Enhancing electrical outputs of the fuel cells with Geobacter sulferreducens by overexpressing nanowire proteins. https://doi.org/10.1111/1751-7915.14128. Microb Biotechnol n/a
Watts MP, Lloyd JR (2013) Bioremediation via microbial metal reduction. In: Microbial metal respiration. Springer, pp 161–201
Wielinga B, Mizuba MM, Hansel CM, Fendorf S (2001) Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environ Sci Technol 35:522–527
Windt W, De, Aelterman P, Verstraete W (2005) Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol 7:314–325
Wong MT, Cheng D, Wang R, Hsing I-M (2016) Modifying the endogenous electron fluxes of Rhodobacter sphaeroides 2.4. 1 for improved electricity generation. Enzyme Microb Technol 86:45–51
Xiang K, Qiao Y, Ching CB, Li CM (2009) GldA overexpressing-engineered E. coli as superior electrocatalyst for microbial fuel cells. Electrochem commun 11:1593–1595
Xiao X, Liu Q-Y, Li T-T et al (2017a) A high-throughput dye-reducing photometric assay for evaluating microbial exoelectrogenic ability. Bioresour Technol 241:743–749. https://doi.org/10.1016/j.biortech.2017.06.013
Xiao Y, Zhang E, Zhang J et al (2017b) Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci Adv 3:1–9. https://doi.org/10.1126/sciadv.1700623
Xu S, Barrozo A, Tender LM et al (2018) Multiheme cytochrome mediated redox conduction through Shewanella oneidensis MR-1 cells. J Am Chem Soc 140:10085–10089
Yalcin SE, Malvankar NS (2020) The blind men and the filament: understanding structures and functions of microbial nanowires. Curr Opin Chem Biol 59:193–201
Yamamura S, Amachi S (2014) Microbiology of inorganic arsenic: from metabolism to bioremediation. J Biosci Bioeng 118:1–9
Yamamura S, Sudo T, Watanabe M et al (2018) Effect of extracellular electron shuttles on arsenic-mobilizing activities in soil microbial communities. J Hazard Mater 342:571–578
Yang Y, Xu M, Guo J, Sun G (2012) Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem 47:1707–1714. https://doi.org/10.1016/j.procbio.2012.07.032
Yang Y, Ding Y, Hu Y et al (2015) Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth Biol 4:815–823
Yang Y, Kong G, Chen X et al (2017) Electricity generation by Shewanella decolorationis S12 without cytochrome c. Front Microbiol 8:1–8. https://doi.org/10.3389/fmicb.2017.01115
Yang Y, Luo O, Kong G et al (2018) Deciphering the anode-enhanced azo dye degradation in anaerobic baffled reactors integrating with microbial fuel cells. Front Microbiol 9:1–10. https://doi.org/10.3389/fmicb.2018.02117
Yang G, Huang L, Yu Z et al (2019) Anode potentials regulate Geobacter biofilms: new insights from the composition and spatial structure of extracellular polymeric substances. Water Res 159:294–301
Ye Y, Liu X, Nealson KH et al (2022) Dissecting the structural and conductive functions of. MBio 13:1–12
Yee MO, Rotaru AE (2020) Extracellular electron uptake in Methanosarcinales is independent of multiheme c-type cytochromes. Sci Rep 10:372
Yi H, Nevin KP, Kim B-C et al (2009) Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens Bioelectron 24:3498–3503
Yong P, Farr JPG, Harris IR, Macaskie LE (2002) Palladium recovery by immobilized cells of Desulfovibrio desulfuricans using hydrogen as the electron donor in a novel electrobioreactor. Biotechnol Lett 24:205–212
Yong Y-C, Yu Y-Y, Li C-M et al (2011) Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biosens Bioelectron 30:87–92
Yong Y-C, Zhong J-J (2012) Impacts of quorum sensing on microbial metabolism and human health. Futur Trends Biotechnol 25–61
Yong X-Y, Feng J, Chen Y-L et al (2014a) Enhancement of bioelectricity generation by cofactor manipulation in microbial fuel cell. Biosens Bioelectron 56:19–25
Yong X-Y, Shi D-Y, Chen Y-L et al (2014b) Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour Technol 152:220–224
Yu Y-Y, Fang Z, Gao L et al (2018) Engineering of bacterial electrochemical activity with global regulator manipulation. Electrochem commun 86:117–120. https://doi.org/10.1016/j.elecom.2017.12.003
Yu H-Q (2020) Molecular insights into extracellular polymeric substances in activated sludge. Environ Sci Technol 54:7742–7750
Yu Y-Y, Cheng Q-W, Sha C et al (2020) Size-controlled biosynthesis of FeS nanoparticles for efficient removal of aqueous cr (VI). Chem Eng J 379:122404
Yurkova NA, Lyalikova NN (1990) New vanadate-reducing facultative chemolithotrophic bacteria. Microbiology 59:672–677
Zhang H, Hu X (2017) Rapid production of pd nanoparticle by a marine electrochemically active bacterium Shewanella sp. CNZ-1 and its catalytic performance on 4-nitrophenol reduction. RSC Adv 7:41182–41189
Zhang B, Cheng HY, Wang A (2021) Extracellular electron transfer through visible light induced excited-state outer membrane C-type cytochromes of Geobacter sulfurreducens. Bioelectrochemistry 138:107683. https://doi.org/10.1016/j.bioelechem.2020.107683
Zhang F, Xu W, Zhang L et al (2022a) Riboflavin as a non-quinone redox mediator for enhanced cr (VI) removal by Shewanella putrefaciens. J Mol Liq 351:118622
Zhang P, Yang C, Li Z et al (2022b) Accelerating the extracellular electron transfer of Shewanella oneidensis MR-1 by carbon dots: the role of carbon dots concentration. Electrochim Acta 421:140490. https://doi.org/10.1016/j.electacta.2022.140490
Zhang T, Bain TS, Nevin KP et al (2012) Anaerobic benzene oxidation by Geobacter species. Appl Environ Microbiol 78:8304–8310
Zhang X (2020) Extracellular electron transport of electroactive biofilm. Bioelectrosynthesis Princ Technol Value-Added Prod 295–305
Zhao J, Li F, Cao Y et al (2021) Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol Adv 53:107682. https://doi.org/10.1016/j.biotechadv.2020.107682
Zheng S, Liu F, Wang B et al (2020) Methanobacterium capable of direct interspecies electron transfer. Environ Sci Technol 54:15347–15354
Zheng T, Xu Y-S, Yong X-Y et al (2015) Endogenously enhanced biosurfactant production promotes electricity generation from microbial fuel cells. Bioresour Technol 197:416–421
Zhong Y, Shi L (2018) Genomic analyses of the quinol oxidases and/or quinone reductases involved in bacterial extracellular electron transfer. Front Microbiol 3029
Zhuang Z, Yang G, Zhuang L (2022) Exopolysaccharides matrix affects the process of extracellular electron transfer in electroactive biofilm. Sci Total Environ 806:150713. https://doi.org/10.1016/j.scitotenv.2021.150713
Acknowledgements
The authors of the manuscript are thankful to the Indian Institute of Technology (BHU) Varanasi, Varanasi, for extending their technical and financial support.
Author information
Authors and Affiliations
Contributions
Manisha Verma writes the manuscript and prepared graphical illustrations. Vishal Singh rechecked, edited the manuscript for grammatical mistakes. Dr. Vishal Mishra has supervised edited and added valuable facts to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, M., Singh, V. & Mishra, V. Moving towards the enhancement of extracellular electron transfer in electrogens. World J Microbiol Biotechnol 39, 130 (2023). https://doi.org/10.1007/s11274-023-03582-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03582-8