Abstract
Artemisinin and its analogues are naturally occurring most effective antimalarial secondary metabolites. These compounds also possess activity against various types of cancer cells, schistosomiasis, and some viral diseases. Artemisinin and its derivatives (A&D) are found in very low amounts in the only natural source i.e. Artemisia plant. To meet the global needs, plant sources have been exploited for the enhanced production of these natural products because their chemical synthesis is not profitable. The generally adopted approaches include non-transgenic (tissue and cell cultures) and transgenic together with the cell, tissue, and whole transgenic plant cultures. The genes targeted for the overproduction of A&D include the biosynthetic pathway genes, trichome development genes and rol genes, etc. Artemisinin is naturally produced in trichomes of leaves. At the same time, transgenic hairy roots are considered a good source to harvest artemisinin. However, the absence of trichomes in hairy roots suggests that artemisinin biosynthesis is not limited to trichomes. Moreover, the expression of the gene involved in trichome development and sesquiterpenoid biosynthesis (TFAR1) in transgenic and non-transgenic roots provokes researchers to look for new insight of artemisinin biosynthesis. Here we discuss and review precisely the various biotechnological approaches for the enhanced biosynthesis of A&D.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artemisinin is an antimalarial pro-drug which has been considered the last line of defence against malaria for many decades (Bryant et al. 2015). Whereas, the other older generation antimalarial drugs are now found less effective due to the acquired resistance against them (Fidock 2010). Artemisinin, a sesquiterpene lactone is the core component of artemisinin-based combination therapies (ACTs) especially for the treatment of multidrug-resistant malaria (Eckstein-Ludwig et al. 2003). With the passage of time, there is a significant rise in the demand for ACTs, as can be estimated from the fact that the number of ACT treatments rose to 36 fold during 2005 to 2013 in endemic countries, reaching a total of 392 million in 2013 (WHO 2017). But the reality seems to be far more challenging when it comes to the reliable supply of artemisinin as the precursor compound for the active ingredient of ACTs. This fact is no doubt of crucial importance in the fight against malaria, which is the fifth most prevalent disease in underdeveloped countries and tenth overall cause of death. This scenario is projected to stay at the same level till 2030 (Nahlen et al. 2005). According to WHO, 216 million cases of malaria occurred globally in 2016 and it led to 445,000 deaths (WHO 2017).
Artemisinin is naturally found in the aerial parts of the plant, i.e. flowers, leaves, stems, buds and seeds (Ferreira et al. 1995a) in amounts ranging from 0.1 to 0.8% of the dry weight of the plant (Abdin et al. 2003), maximally reaching 1.5% in some cases (Kumar et al. 2004; Weathers and Towler 2012). Ontogeny and phenology play important role in the production of secondary metabolites in plants. Its synthesis is basically carried out in the glandular trichomes (GLTs), present on flowers, floral buds, and leaves and sequestered in the subcuticular sac at the apex of the GLTs. Trichome-specific fatty acyl-CoA reductase 1 (TAFR1) is thought to be involved in GLT development and sesquiterpenoid biosynthesis; both are important for artemisinin production (Ferreira et al. 1995a; Olsson et al. 2009).
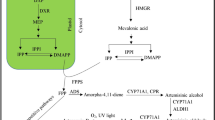
Artemisinin biosynthesis is almost completely known (Nguyen et al. 2011) (Fig. 1). It originates from a common biosynthetic precursor for the synthesis of terpenoids that is isopentenyl diphosphate (IPP), formed via either the cytosolic mevalonate pathway (MVA) or the plastidic mevalonate-independent pathway (MEP); both pathways supply the IPP for artemisinin (Croteau et al. 2000). Condensation of three molecules of IPP to farnesyl diphosphate (FDP) is catalyzed by farnesyl diphosphate synthase (FPS). Amorphadiene synthase (ADS) a sesquiterpene cyclase then catalyzes the formation of amorpha-4,11-diene by the cyclization of FDP. The next two steps are catalyzed by a cytochrome P450, CYP71AV1 (CYP). Amorpha-4,11-diene is oxidized to the artemisinic aldehyde and also to artemisinic acid (AA) (Teoh et al. 2006), which is then converted by a double-bond reductase (DBR2) to dihydroartemisinic aldehyde, the precursor to dihydroartemisinic acid (DHAA) (Zhang et al. 2008). Conversion of DHAA to AN is a non-enzymatic photo-oxidative reaction involving reactive oxygen species (ROS), which by adding three oxygen atoms leads to the formation of the endoperoxide pharmacophore of AN (Brown and Sy 2007; Wallaart et al. 2001).
A schematic diagram showing isopentenyl diphosphate and artemisinin biosynthetic pathway (Arsenault et al. 2010a)
The production of artemisinin is compromised because particularly in third world countries the only reliant source of its production is Artemisia plant with several limitations. First of all, being a competitor against food for the use of land it will cause a rise in food prices thus giving less incentive to farmers for its cultivation. Additionally, the plan for A. annua cultivation has to be designed somehow 14 months before the drugs can be produced. In this regard, natural disasters like flood may be the limiting factor making the artemisinin production unpredictable (Noorden 2010). As far as the chemical synthesis of artemisinin is concerned that has been found to be complex and expensive and thus have not been supplanted as the favored method. Although the chemical synthesis of artemisinin is reported (Zhu and Cook 2012), to date the plant remains the only source of this drug. On the account of all these factors, different setups are being developed for the inexpensive production of artemisinin, which is not reliant on the cultivation of A. annua and should have the capability to get scaled up when required (Table 1).
So far, three different approaches have been practiced for increased production of A&D, including non-transgenic, transgenic and heterologous transgenic systems (Arsenault et al. 2008). Non-transgenic approaches include the selective breeding of high artemisinin-containing elite varieties of A. annua, manipulation of growth condition, use of in vitro cultures, and elicitation to use plant’s natural defence system. In transgenic approach, transgenics of different Artemisia species with a variety of genes have been produced. While, in the heterologous systems, key genes of artemisinin biosynthetic pathway are inserted into organisms other than A. annua. Here we discuss the so far progress made on the biotechnological approaches (transgenic and heterologous transgenic systems) for enhanced artemisinin production.
Non-transgenic approaches
Highly exploited medicinal compounds, artemisinin, and their derivatives are vastly studied for their antimalarial and anticancer effects. So far, precursor molecules of a pathway, intermediate compounds of a pathway or chemicals like methyl jasmonate (MeJ), chitosan and salicylic acid (SA) have been used in a majority of the elicitation studies to enhance a number of secondary metabolites. Non-transgenic approaches are considered easy to practice with low-level ethical constraints. Wild aerial part, in vitro raised plants and greenhouse acclimatized plants of A. amygdalina revealed the presence of artemisinin (Rasool et al. 2013). But, wild inflorescences and calli did not show the presence of artemisinin. Lualon et al. (2008) regenerated untransformed plants of A. annua using 0.1 mg/l thidiazuron (TDZ) and found artemisinin content of 3.36 µg/mg dry weight which is two-fold higher than that of in vitro grown plants of the same age. However, when ex vitro grown untransformed plants of A. annua were elicited with chitosan oligosaccharide (COS) and salicylic acid (SA), COS up-regulated the transcriptional levels of the genes ADS and TTG1 2.5 fold and 1.8 fold after 48 h individually, whereas SA only up-regulated ADS 2.0 fold after 48 h (Yin et al. 2012). Additionally, the supplementation of dimethyl sulfoxide (DMSO) to the untransformed seedlings increased artemisinin in the shoots of A. annua (Mannan et al. 2010b). Rooting of untransformed A. annua shoots (SAM clone) by the supplementation of a-naphthaleneacetic acid increases trichome size on leaves and helps drive the final steps of the biosynthesis of artemisinin (Nguyen et al. 2013).
Baldi and Dixit (2008) reported that elicitation of cell cultures of A. annua (untransformed) with mevalonic acid (MA) (50 mg/L) resulted maximally an increase of twofold of artemisinin content in comparison to control, while a maximum increase of 3.47 fold in artemisinin was attained when MeJ (5 mg/L) was added. Combined supplementation of MA and MeJ resulted in maximum artemisinin production of 96.8 mg/L which was 4.79 times higher than control callus cultures (20.2 mg/L). However, some researchers suggest that elicitation might not be a good method to enhance artemisinin because it triggered biosynthesis after a long time (48 h). Another study conducted on A. annua suggests roots (which neither produce significant artemisinin nor its precursor compounds) to regulate artemisinin production in the leaves. Researchers grafted roots of lines with high artemisinin-producing leaves to the low artemisinin producing shoots and observed an increased leaf production of artemisinin in those low artemisinin-producing plants (Wang et al. 2016). A wide range of various reports describing the A&D production and its uses is given below.
Transgenic approaches
Transformation with genes involved in artemisinin biosynthetic pathway (ABP)
Transgenic Artemisia
Modifications have been made in plants by improving the expression of endogenous pathways or by introducing novel genes to modify its pathways (Ikram and Simonsen 2017). Researchers from numerous groups have almost resolved the difficult task of biosynthesis of artemisinin. Now, noticeable progress has been observed in the regulation of biosynthesis of artemisinin and underlying mechanisms (Bouwmeester et al. 1999). Key enzymes and their genes which are main precursors and are necessary for biosynthesis of artemisinin i.e. [farnesyl diphosphate synthase (FPS), amorpha-4,11-diene synthase (AMS)], and the genes of the enzymes linked with the molecular basis of biosynthesis of artemisinin including squalene synthase (SQS), have been cloned from A. annua (Matsushita et al. 1996; Mercke et al. 2000; Wallaart et al. 2001; Yan et al. 2003). Dhingra and Narasu (2001) isolated the main enzyme used in the biochemical conversion of arteannuin B to artemisinin. The genes of artemisinin biosynthesis including ADS, CYP71AV1, DBR2, and ALDH1 are all preferably articulated in the glandular trichomes (Covello et al. 2007; Olsson et al. 2009).
Several groups have shown that transformation with artemisinin biosynthetic pathway genes can increase artemisinin production through modification of its biosynthetic pathway (Ikram and Simonsen 2017). Overexpression of FPS in A. annua leads to the accumulation of the increased amount of artemisinin through conversion of IPP and DMADP into FDP (Maes et al. 2011). Biosynthesis of artemisinin can be improved significantly by an enhanced expression of both HMGR and ADS instantaneously through a co-transformation experiment in A. annua (Alam and Abdin 2011); furthermore, production of artemisinin can also be improved by overexpressing both HMGR and FPS together in A. annua (Wang et al. 2011). In the MEP pathway, DXR has been considered as an important rate-limiting enzyme effectively used to increase the production of monoterpenoids such as peppermint essential oils (Hasunuma et al. 2008). Genetic map of A. annua further proved that DXR was strongly interconnected with the production of artemisinin (Graham et al. 2010). Chen et al. (1999) raised hairy roots of A. annua plants after the infection of engineered A. rhizogenes containing fds gene. The transgenic hairy roots showed an overexpression of FPS and an improved production of artemisinin content i.e. 3 to fourfold higher than control. Further, Chen et al. (2000) reported 2 to threefold increase in artemisinin production in A. annua plants transformed with same FPS gene through A. tumefaciens mediated genetic transformation method.
The cloning and characterization of AaHDR involved in the increased production of artemisinin through enhanced production of artemisinin biosynthesis precursors via MEP pathway in A. annua has been reported (Peng et al. 2011). It will be helpful to understand more about the function of HDR at the level of molecular genetics and unveil the biosynthetic mechanism of artemisinin. Genetic engineering can be used for enhanced artemisinin production through an increase in expression of the key genes of the enzymes used for the artemisinin biosynthesis, such as FPS and AMS, in the transgenic high-yield A. annua.
As chemical synthesis of A&D is not economically feasible (Delabays et al. 2001), wild plants contain scarce amount of these secondary metabolites (Mannan et al. 2010a) and ~ 40% of the global population is threatened by malaria (Dhingra et al. 1999), there is a high demand for these compounds in the international market. To satisfy the vast need for medicine, various transgenic biotechnological approaches are discussed here (Fig. 2).
Heterologous systems
Progressive achievements in molecular biology enabled scientists to explore new expression systems for artemisinin biosynthesis beyond its only source on the planet earth i.e. A. annua. Genetic engineering of the molecular pathway(s) leading to the synthesis of artemisinin has been attempted in several organisms including plants, yeast, and bacteria to improve its production. Arsenault et al. (2008) has explained in detail different approaches using heterologous expression systems for the enhanced production of artemisinin and focused on newer methodologies of genetic engineering of artemisinin biosynthetic pathway genes to meet the demands for the treatment of different diseases especially malaria.
Heterologous expression system especially Escherichia coli and Saccharomyces cerevisiae have been studied so far for this purpose (Arsenault et al. 2008). It is a promising technology for the production of important compounds; however, monomeric proteins can be easily produced in microbes while the production of complex secondary metabolites needs the reconstruction of the metabolic pathway. Various approaches have been developed to express the sesquiterpene lactones in microbial systems including fungi (Asadollahi et al. 2008) and bacteria (Picaud et al. 2007). However, there are few reports describing the cloning of genes into microbial hosts specifically for the biosynthesis of artemisinin and its derivatives. Majority of these reports reveal the expression of amorpha-4,11-diene which is a volatile precursor of artemisinin. In a report, a 9-genes biosynthetic pathway of amorpha-4,11-diene was introduced in E. coli. Keeping in mind the loss of gaseous phase, microbes were cultured in a two-phase partitioning bioreactor and 89% pure amorpha-4,11-diene was procured in ~ 0.5 g/L concentration of the culture medium (Newman et al. 2006). A semi-synthetic approach of artemisinin by using strains of S. cerevisiae for biological production of artemisinic acid and its chemical conversion to artemisinin is also reported (Paddon et al. 2013). Ro et al. (2006) engineered S. cerevisiae mevalonate pathway with amorphadiene synthase (ADS) and cytochrome P450 monooxygenase (CYP71AV1) to produce artemisinic acid. They found higher levels of artemisinic acid (up to 100 mg/L) in reconstructed yeast than A. annua. Further, a cDNA clone was generated from the mRNA of trichomes encoding cytochrome P450 (CYP71AV1) and expressed in S. cerevisiae which catalyzed the oxidation of various intermediates of artemisinin biosynthetic pathway including amorpha-4,11-diene (Teoh et al. 2006). Although there are some successful reports for the production of various secondary metabolites in reconstructed microbes, the expression of complex pathway limits the heterologous expressions of the non-native molecule (Newman et al. 2006).
Transformation with genes not involved in artemisinin biosynthetic pathway (ABP)
An efficient methodology to genetically target the desired plant metabolites is the metabolic engineering, which is also a hot spot for genetic engineering of different medicinal plants. Different attempts have been taken to increase artemisinin production in Artemisia species through the insertion of different genes affecting flowering (Wang et al. 2007), phytohormone levels (Sa et al. 2001) and farnesyl diphosphate synthesis (Chen et al. 2000).
Transformation with flowering genes
Artemisia species were transformed with different flowering stimulating genes because it has been established that artemisinin production is higher during the flowering season. Wang et al. (2007) has transformed A. annua with an early flowering gene CONSTANS (CO) and the flowering promoting factor 1 gene (fpf1), both from Arabidopsis, using A. tumefaciens. Though fpf1 transformed plants showed flowering 20 days earlier than non-transgenic plants, yet no major difference in artemisinin production was observed. CO transformed plants also showed the similar results with early flowering in transformed plants but no significant increase in artemisinin concentration depicting direct relation between flowering and production of artemisinin.
Transformation with genes of phytohormones
Genes encoding phytohormone or phytohormone were also found triggering enhanced production of A&D. Transformation of A. annua plants through A. tumefaciens containing the Isopentenyl transferase gene (ipt) resulted in 70% increase of artemisinin concentration than control with an increase in chlorophyll content as well (Sa et al. 2001). Likewise, Singh et al. (2016) studied the effect of expression of β-glucosidase gene (bgl1) in A. annua and found that bgl1 induced trichome density (up to 20% in leaves and 66% in flowers) and artemisinin content (up to 1.4% in leaf and 2.56%/g dry weight in flowers) of transgenic plants than the control plants.
Transformation with the genes of trichome development
Artemisinin is produced in glandular trichomes, which are about 10-cells in size located on leaves, floral buds, and flowers (Ferreira et al. 1995b; Olsson et al. 2009; Tan et al. 2015; Tellez et al. 1999) and sequestered in the epicuticular sac which is present at the apex of the trichome (Olsson et al. 2009). Presence of trichomes in aerial parts of Artemisia species and their absence in roots supports the higher accumulation of artemisinin in the aerial parts of Artemisia species (Fig. 3). For example, the leaves which are younger and later in growth level have the higher amount of artemisinin as compared to the mature leaves formed during the early stages of plant’s development; this difference was accounted for higher trichome density and a higher capacity per trichome in the upper leaves (Lommen et al. 2006). Trichome-specific fatty acyl-CoA reductase 1 (TFAR1) is known to stimulate trichome development and to catalyze sesquiterpenoid biosynthesis (Maes et al. 2011). Dilshad et al. (2015b) supported the idea of overexpression of TFAR1 genes and hence artemisinin production. Another report by Dilshad et al. (2015a) explained the effect of a higher expression of TFAR1 gene in A. carvifolia is directly linked to the enhanced production of artemisinin. There are several other reports on overexpression of trichome genes and enhanced production of artemisinin. Tan et al. (2015) transformed A. annua with TRICHOME AND ARTEMISININ REGULATOR 1 (TAR1) gene and observed a significant increase in artemisinin production and also suggested that this gene is directly linked to artemisinin pathway genes at some stages of development. Besides TFAR1 and TAR1 transformations, Liu et al. (2009) reported that amount of glandular trichomes can be improved by overexpressing putative TTG1 gene related to significant increase in artemisinin production in A. annua leaves. Overexpression of an important trichome-specific transcription factor (AaORA) in A. annua both glandular secretory trichomes and nonglandular T-shaped trichomes (TSTs) is highly expressed in transformed lines and acts as a positive regulator in the biosynthesis of artemisinin (Lu et al. 2013).
Trichomes present on leaf surfaces of Artemisia species (a–c), while transgenic hairy roots are devoid of trichomes (d). a Fluorescent microscopy of leaf of transgenic A. annua containing rol genes, b environmental scanning electron microscopy of the leaf of transgenic A. annua harboring rol genes, c confocal microscopy of leaf of transgenic A. annua containing rol genes, d environmental scanning election microscopy of leaf of untransformed in vitro grown A. annua
rol genes
Hairy roots raised through the infection of Agrobacterium rhizogenes
Hairy roots syndrome appears after the infection of Agrobacterium rhizogenes, which induces the plagiotropic roots having increased growth rate and multiple ramifications. Transgenic hairy roots are somewhat similar in morphology to the normal untransformed roots and they have been raised for the commercial-scale production of secondary metabolites (Sharma et al. 2013). In early studies, Weathers et al. (1994) obtained several transgenic root cultures of A. annua from the infection of A. rhizogenes strain ATCC 15834 and found the biosynthesis of artemisinin up to 0.42% of dry weight (DW). They also detected artemistene, artemisinic acid and arteannuin B and suggested that the commercial production of these compounds using transformed roots is feasible. A rapidly growing clone (YUT16) of these hairy roots (diploid) was used to produce four stable tetraploid clones of A. annua. These tetraploid clones revealed a better growth rate and produced up to six times more artemisinin than the diploid parent suggesting the importance of ploidy level in artemisinin biosynthesis (De Jesus-Gonzalez and Weathers 2003). Further, the diploid clones of YUT16 were grown in nutrient mist reactors and resulted in an increase of ~ 3 times as much artemisinin (2.64 µg/g DW) as roots grown in bubble column reactors (0.98 µg/g DW) (Kim et al. 2001). When cultures of this hairy root line were grown in continuous light, they showed a substantial increase in deoxy-d-xylulose-5-phosphate synthase (DXPR) transcript levels compared to dark-grown cultures suggesting plastid-localized artemisinin biosynthetic pathway for its higher biosynthesis (Souret et al. 2002). Growth kinetics study of these roots (Weathers et al. 1994) showed that the use of gibberellic acid (GA3) at 0.01 mg/L (~ 28.9 µM) increased the growth rate of hairy roots of A. annua by 24.9% (Smith et al. 1997). This genetically stable roots clone (YUT16) reveals that there are many factors like the ploidy level, reactors type, position of the roots, presence or absence of light and elicitors which effect artemisinin production.
Hairy roots of in vitro generated A. annua plants were also obtained by Ahlawat et al. (2012) with the infection of A. rhizogenes strain LBA-9402 and revealed maximum artemisinin volumetric biosynthesis of 390 µg/L/d. In another report, transformed root cultures of A. annua established by the infection of A. rhizogenes strain LBA 9402 revealed production of artemisinin up to 0.004% dry weight (DW). However, supplementation of GA3 in medium provoked artemisinin biosynthesis up to 400% of the control value (Paniego and Giulietti 1996).
Besides artemisinin, its other derivatives have also been studied in transgenic hairy root cultures of Artemisia species. Banerjee et al. (1997) infected the leaves of A. annua with A. rhizogenes strain LBA 9402 and the leaf developed hairy roots which have the ability to produce artemisinic acid and arteannuin B. Interestingly, they found that regenerants of transgenic hairy roots represented a higher growth rate and produced more of these secondary metabolites than the respective mother hairy root clone. In another attempt, transgenic hairy roots of A. annua were obtained from NCIB 8196 or MAFF 03-01724 strain of A. rhizogenes. Among them, some of the clones were grown in dark, produced undetectable level while some clones cultured in light and in liquid medium produced signals of artemisinin in GC-MS analysis (Jazir et al. 1995). Similarly, Shaneeja et al. (2014) has reported an improved amount of artemisinin in hairy roots of A. annua obtained after the infection of two different A. rhizogenes species.
Being the intrinsic property of Artemisia species to produce artemisinin, other species of Artemisia have also been exploited to harvest artemisinin. Mannan et al. (2008) raised transgenic hairy roots by infecting the plants of A. dubia and A. indica with A. rhizogenes strains LBA 9402 and 8196. Hairy roots induction was found higher in infected with LBA9402 as compared to 8196. However, roots of A. dubia infected either with LBA9402 or 8196 produced maximum artemisinin (0.603% and 0.753% of DW, respectively). Contrary to it, relatively lower amount of artemisinin was harvested in in vitro regeneration of A. indica hairy roots obtained by the infection of strain 8196 in the liquid medium. Those in vitro raised hairy roots resulted in highest root fresh weight as well as artemisinin content (3.9 g and 0.042%, respectively). However, transgenic hairy roots obtained from A. dubia explants with the infection of A. rhizogenes strain 9402 revealed a higher growth rate (twofold) and a higher production of artemisinin content (36.581 µg/g DW) as compared to control untransformed roots which did not show any artemisinin content (Kiani et al. 2012).
Elicitation of the hairy roots
Elicitors or signal molecules are usually the chemicals that modify cell metabolism in order to enhance the biosynthesis of secondary metabolites in a plant cell or tissue cultures. They enable plants in better adaptation to the stress conditions. Certain elicitors have been employed to the cell or hairy root culture of Artemisia species for enhanced production of artemisinin and its derivatives. Weathers et al. (1997) studied the effect of four different factors to optimize conditions to harvest maximum root biomass and terpenoid production in transgenic A. annua hairy root clones (YUT16). They found that 15 mM nitrate, 1.0 mM phosphate, 5% sucrose content (wt/vol) and 8 days old cultured roots are the best conditions to harvest maximum biomass as well as terpenoid content.
Besides the above-optimized factors, other elicitors/signal molecules have been used to raise the A&D amount in transgenic hairy roots of Artemisia species. Hairy roots induced by the leaf disc method of A. annua showed an enhanced production of artemisinin (550 mg/L) when elicited with a homogenate of Aspergillus oryzae (Liu et al. 1997). In another report, diploid clones of highly exploited YUT16 hairy roots obtained from A. annua (Weathers et al. 1994) when subjected to measure the effect of a broad range of phytohormones on growth of A. annua hairy roots and their artemisinin content which revealed that GA3 (0.029 µM) produced the highest values of growth while 2-isopentenyladenine triggered artemisinin biosynthesis more than twice that of the B5 controls, and more than any other hormone studied (Weathers et al. 2005). Hairy roots of A. annua obtained from the seeds of YU strain represented sustained and rapid growth when conditioned medium was fed to the roots and the presence of 1% CO2 in the carrier gas did not enhance the growth kinetics but it did prevent necrosis of the tissue at the highest mist cycle (Wang and Weathers 2007). GA3 also affected the A&D production in other species of Artemisia. Hairy root culture of A. dubia obtained by the infection of A. rhizogenes strain LBA9402 represented highest artemisinin accumulation of 80 ± 3 µg/g of DW (91% increase) when cultured on media containing GA3 (0.001 mg/L) while 79 ± 3 µg/g of DW was found at 0.138 mg/L of salicylic acid separately as compared to control (Ali et al. 2012).
Supplementation of growth medium with different carbon sources was also found affecting A&D production. It is found that glucose-stimulated artemisinin production maximally in diploid and tetraploid clones of YUT16 hairy root strain while growth in sucrose and fructose was significantly better than in glucose (Weathers et al. 2004). A. annua seedlings obtained from the seeds of YU strain produced a 200% increase in artemisinin content by glucose as a carbon source in the medium as compared to sucrose and suggested that these sugars also act as signals to affect the downstream production of artemisinin (Wang and Weathers 2007). The biosynthesis of artemisinin is increased in response to exogenous glucose supplementation in A. annua seedlings obtained from the seeds of YU cultivar of A. annua by upregulating FPS, DXS, DXR, ADS and CYP transcript levels (Arsenault et al. 2010b). Weathers et al. (2012) found that monosaccharides i.e. glucose increased artemisinin biosynthesis in both the seedlings and hairy root cultures of A. annua plants by increasing the expression of some of the genes in the artemisinin biosynthetic pathway. However, disaccharides were found inhibiting the artemisinin biosynthesis which suggests that monosaccharides play a dual role; not only fulfill the carbon requirement for plants but also as a signal switching on signal transduction of artemisinin biosynthetic pathway.
Hairy roots in different bioreactors
Different bioreactors have been used to grow plant cells (transformed and untransformed) and this technology has been adopted on a wide scale for the production of A&D. The hairy root cultures obtained from leaf discs (Liu et al. 1997) of A. annua were cultivated in a flask, a bubble column, a modified bubble column and a modified inner-loop airlift bioreactor and artemisinin contents in the latter two culture conditions were found higher among the four i.e. 536 mg/L after 20 days (Liu et al. 1998). Highly studied hairy root clone YUT16 obtained by Weathers et al. (1994) was grown at 1 L in disposable culture bag mist reactor showed growth rates higher than that of shake flasks (Sivakumar et al. 2010). Although expression in reactors was equivalent to or greater than that of root cultures of YUT16 grown in shake flasks, surprisingly, transcriptional regulation of HMGR, DXS, DXR, and FPS was greatly affected by the position of the roots in each reactor (Souret et al. 2003).
rol genes carrying transgenic plants
Over the past decade, rolA, rolB and rolC genes have shown to possess the property of overproduction of secondary metabolites in transformed plant cells. Plants growth and metabolism appeared to be associated with the rolA protein, which acts as a stimulator of secondary metabolism (Altvorst et al. 1992; Schmülling et al. 1993). The rolB protein plays an important role in the pathway of signal transduction of auxin due to its tyrosine phosphatase activity (Filippini et al. 1996). Estruch et al. (1991) described that rolC has cytokinin glucosidase activity and it can be associated with the release of active cytokinins from their inactive glucosides. RolC has also been shown to be involved in the production of tropane alkaloids (Bonhomme et al. 2000), pyridine alkaloids (Palazón et al. 1998b), indole alkaloids (Palazón et al. 1998a), ginsenosides (Bulgakov 2008) and anthraquinones (Bulgakov et al. 2002, 2003; Shkryl et al. 2008) in transformed plants and plant cell cultures. Regarding the rolD gene, its main function is the conversion of ornithine to proline due to its ornithine cyclodeaminase enzyme activity (Trovato et al. 2001).
Numerous reports have shown that hairy roots cultures can produce a significant amount of secondary metabolites in different plant species transformed with Agrobacterium rol genes (Giri and Narasu 2000; Oksman-Caldentey and Sévon 2002). There are few reports about the transformation of Artemisia with rol genes for production of artemisinin. Our group is working in this direction for several years, and we are successfully producing artemisinin through rol genes transformation of different Artemisia species using A. tumefaciens and A. rhizogenes.
Using Agrobacterium tumefaciens mediated genetic transformation technology, A. carvifolia was transformed with GV3101 strain harboring rolB and rolC genes for enhanced production of artemisinin and its derivatives. Artemisinin content was increased from 3 to sevenfold in transgenics transformed with the rol B gene, and 2.3 to sixfold in those transformed with the rolC gene (Dilshad et al. 2015a). We have also shown a 2 to ninefold increase in artemisinin content of A. annua plants transformed with rolB genes and about fourfold increase in artemisinin amount in rolC genes transformed plants (Dilshad et al. 2015b). Besides this, an increase in artemisinin content of A. dubia transformed with rolABC genes using A. tumefaciens has been reported (Kiani et al. 2012). The rol genes were found to overexpress intermediate pathway genes of artemisinin biosynthetic pathway. This idea can be supported by Kiani et al. (2016) who transformed Artemisia annua and A. dubia with rolABC genes and the leaves of transgenic plants revealed an increase in artemisinin content up to ninefold when compared to untransformed plants. Interestingly, transgenic plants expressed CYP71AV1 and ALDH1 levels higher than that of ADS. Moreover, the level of the TFAR1 expression and trichome density was also significantly increased in all transgenic lines.
We transformed different Artemisia plants with rol genes which are responsible for enhancing production of secondary metabolites in plants. The exact mechanisms for the action of the rol genes are not yet clear (Bulgakov 2008) but it has been suggested that they act through the stimulation of the plant’s defense response which includes induction of many of the hormonal pathways. This may explain why the transformation with Agrobacterium rol genes has such a significant effect on the amount of artemisinin produced by the transformed plants. Stimulation of the synthesis of artemisinin within these plants has also allowed us to alter our perception of how and where artemisinin is produced within the plants. This report might help to develop better strategies to increase the production of this valuable therapeutic drug, which will, in turn, allow greater use of it in the chemotherapy of malaria and other diseases.
Conclusions and perspectives
Artemisinin, nowadays, seems to be the only reliable treatment for multidrug-resistant malaria. Millions of deaths of malarial patients demand a reliable supply of artemisinin, which is intrinsically biosynthesized in Artemisia species especially in the trichomes present on the leaves. Due to the limitations of the chemical synthesis of artemisinin, different approaches have been practiced for enhanced production of artemisinin including generation of high artemisinin-containing elite varieties of A. annua, manipulation of growth condition for the plant tissues, use of in vitro cultures and elicitation to use plant’s natural defence system. However, various transgenic approaches were also developed to enhance artemisinin production including generation of hairy root cultures. Hairy roots revealed better growth rate and artemisinin production and unveiled many factors affecting A&D production like the ploidy level, reactors type, position of the roots, presence or absence of light, different strains of A. rhizogenes and elicitors. Elicitors have also been used to increase the artemisinin biosynthesis either alone or in combinations. Various elicitors have been tried to enhance A&D biosynthesis including GA3, COS, DMSO, nitrates, phosphates, sucrose, TDZ, MeJ, chitosan, SA, and MA and found maximally increasing ~ twofold of artemisinin by the transcriptional upregulation of ADS and TTG1. Interestingly, glucose rather than sucrose was found not only fulfilling the carbon requirement but also driving the signal transduction of artemisinin biosynthetic pathway via upregulation of FPS, DXS, DXR, ADS and CYP genes. Transgenic roots clones, either elicited or unelicited, differed in A&D production in the different bioreactors e.g. flask, a bubble column, a modified bubble column and a modified inner-loop airlift bioreactor, nutrient mist reactors and bubble column reactors. The difference in bioreactors also affected transcriptional regulation of HMGR, DXS, DXR, and FPS differently.
Artemisia species have been transformed with a variety of artemisinin biosynthetic pathway genes (FPS, HMGR, ADS, FDS, AMS, SQS, and AaHDR), trichomes development genes (TTG1, AaORA and TAR1) and their overexpression were found increasing A&D production. Majority of these genes either help in conversion of IPP and DMADP into FDP or regulate MEP pathway. However, ADS, CYP71AV1, DBR2, and ALDH1 are preferably expressed in the glandular trichomes. Various Artemisia clones were transformed with the genes other than artemisinin biosynthetic pathway including early flowering gene CO, Ipt and fpf1. However, early flowering did not increase artemisinin concentration but ipt resulted in an increase in 70% of artemisinin concentration. rol genes, on the other hand, holds the property of overproduction of secondary metabolites in transformed cells. They are playing role in signal transduction of auxin and reported for the enhancement of a vast variety of secondary metabolites. There are few reports regarding the transformation of Artemisia with rol genes for enhanced production of artemisinin. Our group is working in this direction for several years and we are successfully producing artemisinin through rol genes transformation of different Artemisia species using A. tumefaciens and A. rhizogenes. rol genes induced an overexpression of TFAR1 in A. annua and A. carvifolia and resulted in enhanced production of artemisinin. rolB gene is reported as the most powerful inducer of secondary metabolism and artemisinin content was found increased up to 7–9 fold in rolB transgenics as compared to rolC transgenic plants. Interestingly, transgenic plants expressed higher transcript levels of CYP71AV1, ADS, and ALDH1 which can be compared to higher TFAR1 expression and trichome density. However, heterologous expression systems for A&D biosynthesis have been studied in E. coli and S. cerevisiae. Majority of these reports reveals the expression of amorpha-4,11-diene, a volatile precursor of artemisinin. In another semi-synthetic approach, S. cerevisiae is used for the production of artemisinic acid and its chemical conversion to artemisinin. Expression of complex pathway declines the heterologous expressions of the non-native molecule. Stimulation of the synthesis of artemisinin within Artemisia plants has also allowed us to alter our perception of how and where artemisinin is produced within the plants. This report might help to develop better strategies to increase the production of this valuable therapeutic drug, which will, in turn, allow greater use of it in the chemotherapy of malaria and other diseases.
Abbreviations
- DXR :
-
1-Deoxy-d-xylulose 5-phosphate reductoisomerase
- DXS :
-
1-Deoxy-d-xylulose 5-phosphate synthase
- ALDH1:
-
Aldehyde dehydrogenase 1
- AMS:
-
Amorpha-4,11-diene synthase
- ADS:
-
Amorphadiene synthase
- AaHDR :
-
Artemisia annua 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase
- AA:
-
Artemisinic acid
- A&D:
-
Artemisinin and its derivatives
- ACTs:
-
Artemisinin based combination therapies
- AN production:
-
Artemisinin production
- COS:
-
Chitosan oligosaccharide
- CO:
-
CONSTANS
- CYP:
-
Cytochrome
- DXPR:
-
Deoxy-d-xylulose-5-phosphate synthase
- DHAA:
-
Dihydroartemisinic acid
- DMSO:
-
Dimethyl sulfoxide
- DMADP:
-
Dimethylallyl diphosphate
- DBR2:
-
Double bond reductase-2
- DW:
-
Dry weight
- FPS:
-
Farnesyl diphosphate synthase
- FDP:
-
Farnesyl diphosphate
- fpf1 :
-
Flowering promoting factor 1 gene
- GA3:
-
Gibberellic acid
- GSTs:
-
Glandular secretory trichomes
- GLTs:
-
Glandular trichomes
- HMGR:
-
Hydroxymethyl glutaryl coenzyme A reductase
- IPP:
-
Isopentenyl diphosphate
- ipt :
-
Isopentenyl transferase gene
- MeJ:
-
Methyl jasmonate
- MEP:
-
Mevalonate independent pathway
- MVA:
-
Mevalonate pathway
- MA:
-
Mevalonic acid
- TSTs:
-
Non-glandular T-shaped trichomes
- ROS:
-
Reactive oxygen species
- rol genes:
-
Root locus genes
- SA:
-
Salicylic acid
- SQS:
-
Squalene synthase
- TDZ:
-
Thidiazuron
- TAR1:
-
TRICHOME AND ARTEMISININ REGULATOR 1
- TFAR1:
-
Trichome-specific fatty acyl-CoA reductase 1
- WHO:
-
World Health Organization
References
Abad MJ, Bedoya LM, Apaza L et al (2012) The Artemisia L. Genus: a review of bioactive essential oils. Molecules 17:2542–2566
Abdin M, Israr M, Rehman R et al (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:289–299
Ahlawat S, Saxena P, Ram M et al (2012) Influence of Agrobacterium rhizogenes on induction of hairy roots for enhanced production of artemisinin in Artemisia annua L. plants. Afr J Biotechnol 11:8684–8691
Ahmad W, Hasan A, Abdullah A et al (2010) Medicinal importance of Artemisia absinthium Linn (Afsanteen) in Unani medicine: a review. Hippocrat J Unani Med 01:117–125
Alam P, Abdin M (2011) Over-expression of HMG-CoA reductase and amorpha-4, 11-diene synthase genes in Artemisia annua L. and its influence on artemisinin content. Plant Cell Rep 30:1919–1928
Ali M, Kiani B, Mannan A et al (2012) Enhanced production of artemisinin by hairy root cultures of Artemisia dubia. J Med Plants Res 6:1619–1622
Altvorst vAC, Bino R, Van Dijk A et al (1992) Effects of the introduction of Agrobacterium rhizogenes rol genes on tomato plant and flower development. Plant Sci 83:77–85
Arsenault PR, Wobbe KK, Weathers PJ (2008) Recent advances in artemisinin production through heterologous expression. Curr Med Chem 15:2886
Arsenault PR, Vail D, Wobbe KK et al (2010a) Reproductive development modulates gene expression and metabolite levels with possible feedback inhibition of artemisinin in Artemisia annua. Plant Physiol 154:958–968
Arsenault PR, Vail DR, Wobbe KK et al (2010b) Effect of sugars on artemisinin production in Artemisia annua L.: transcription and metabolite measurements. Molecules 15:2302–2318
Asadollahi MA, Maury J, Møller K et al (2008) Production of plant sesquiterpenes in Saccharomyces cerevisiae: effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol Bioeng 99:666–677
Baldi A, Dixit V (2008) Enhanced artemisinin production by cell cultures of Artemisia annua. Curr Trends Biotechnol Pharm 2:341–348
Banerjee S, Zehra M, Gupta M et al (1997) Agrobacterium rhizogenes-mediated transformation of Artemisia annua: production of transgenic plants. Planta Med 63:467–469
Bhakuni R, Jain D, Sharma R et al (2001) Secondary metabolites of Artemisia annua and their biological activity. Curr Sci 80:35–48
Bonhomme V, Laurain-Mattar D, Fliniaux M (2000) Effects of the rolC gene on hairy root: induction development and tropane alkaloid production by Atropa belladonna. J Nat Prod 63:1249–1252
Bouwmeester HJ, Wallaart TE, Janssen MH et al (1999) Amorpha-4, 11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry 52:843–854
Brown GD, Sy L-K (2007) In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron 63:9548–9566
Bryant L, Flatley B, Patole C et al (2015) Proteomic analysis of Artemisia annua—towards elucidating the biosynthetic pathways of the antimalarial pro-drug artemisinin. BMC Plant Biol 15:175
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Bulgakov V, Tchernoded G, Mischenko N et al (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97:213–221
Bulgakov V, Tchernoded GK, Mischenko N et al (2003) Effects of Ca2+ channel blockers and protein kinase/phosphatase inhibitors on growth and anthraquinone production in Rubia cordifolia callus cultures transformed by the rolB and rolC genes. Planta 217:349–355
Chen D-H, Liu C-J, Ye H-C et al (1999) Ri-mediated transformation of Artemisia annua with a recombinant farnesyl diphosphate synthase gene for artemisinin production. Plant Cell Tissue Org Cult 57:157–162
Chen D-H, Ye H-C, Li G-F (2000) Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Covello PS, Teoh KH, Polichuk DR et al (2007) Functional genomics and the biosynthesis of artemisinin. Phytochemistry 68:1864–1871
Croteau R, Kutchan T, Lewis N (2000) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, R J (eds) Biochemistry & molecular biology of plants, vol 24. American Society of Plant Physiologists, Rockville, pp 1251–1318
De Jesus-Gonzalez L, Weathers P (2003) Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21:809–813
Delabays N, Simonnet X, Gaudin M (2001) The genetics of artemisinin content in Artemisia annua L. and the breeding of high yielding cultivars. Curr Med Chem 8:1795–1801
Dhingra V, Narasu ML (2001) Purification and characterization of an enzyme involved in biochemical transformation of arteannuin B to artemisinin from Artemisia annua. Biochem Biophys Res Commun 281:558–561
Dhingra V, Rao KV, Narasu ML (1999) Current status of artemisinin and its derivatives as antimalarial drugs. Life Sci 66:279–300
Dilshad E, Cusidò RM, Estrada KR et al (2015a) Genetic transformation of Artemisia carvifolia Buch with rol genes enhances artemisinin accumulation. PLoS ONE 10:e0140266
Dilshad E, Cusidò RM, Palazòn J et al (2015b) Enhanced artemisinin yield by expression of rol genes in Artemisia annua. Malar J 14:1–10
Eckstein-Ludwig U, Webb R, Van Goethem I et al (2003) Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957–961
Estruch J, Chriqui D, Grossmann K et al (1991) The plant oncogene rolC is responsible for the release of cytokinins from glucoside conjugates. EMBO J 10:2889–2895
Ferreira J, Simon J, Janick J (1995a) Relationship of artemisinin content of tissue-cultured, greenhouse-grown, and field-grown plants of Artemisia annua. Planta Med 61:351–355
Ferreira J, Simon JE, Janick J (1995b) Developmental studies of Artemisia annua: flowering and artemisinin production under greenhouse and field conditions. Planta Med 61:167–170
Fidock DA (2010) Drug discovery: priming the antimalarial pipeline. Nature 465:297–298
Filippini F, Rossi V, Marin O et al (1996) A plant oncogene as a phosphatase. Nature 379:499–500
Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18:1–22
Graham IA, Besser K, Blumer S et al (2010) The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science 327:328–331
Hasunuma T, Takeno S, Hayashi S et al (2008) Overexpression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase gene in chloroplast contributes to increment of isoprenoid production. J Biosci Bioeng 105:518–526
Ikram NK, Simonsen HT (2017) A review of biotechnological artemisinin production in plants. Front Plant Sci 8:1966
Ivanescu B, Miron A, Corciova A (2015) Sesquiterpene lactones from Artemisia genus: biological activities and methods of analysis. J Anal Methods Chem 501:247685
Jazir M, Shimomura K, Yoshimatsu K et al (1995) Establishment of normal and transformed root cultures of Artemisia annua L. for artemisinin production. J Plant Physiol 145:175–177
Kavishe RA, Koenderink JB, Alifrangis M (2017) Oxidative stress in malaria and artemisinin combination therapy: Pros and Cons. FEBS J 284:2579–2591
Kiani BH, Safdar N, Mannan A et al (2012) Comparative Artemisinin analysis in Artemisia dubia transformed with two different Agrobacteria harbouring rolABC genes. Plant Omics 5:386–391
Kiani BH, Suberu J, Mirza B (2016) Cellular engineering of Artemisia annua and Artemisia dubia with the rol ABC genes for enhanced production of potent anti-malarial drug artemisinin. Malar J 15:252
Kim Y, Wyslouzil B, Weathers P (2001) A comparative study of mist and bubble column reactors in the in vitro production of artemisinin. Plant Cell Rep 20:451–455
Kim Y, Wyslouzil BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10
Krungkrai J, Krungkrai SR (2016) Antimalarial qinghaosu/artemisinin: the therapy worthy of a Nobel Prize. Asian Pac J Trop Biomed 6:371–375
Kumar S, Gupta S, Singh P et al (2004) High yields of artemisinin by multi-harvest of Artemisia annua crops. Ind Crops Prod 19:77–90
Liu C, Wang Y, Ouyang F et al (1997) Production of artemisinin by hairy root cultures of Artemisia annua L. Biotechnol Lett 19:927–929
Liu C, Wang Y, Ouyang F et al (1998) Production of artemisinin by hairy root cultures of Artemisia annua L. in bioreactor. Biotechnol Lett 20:265–268
Liu C, Zhao Y, Wang Y (2006) Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biotechnol 72:11–20
Liu S, Tian N, Li J et al (2009) Isolation and identification of novel genes involved in artemisinin production from flowers of Artemisia annua using suppression subtractive hybridization and metabolite analysis. Planta Med 75:1542–1547
Liu B, Wang H, Du Z et al (2011) Metabolic engineering of artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep 30:689–694
Lommen W, Schenk E, Bouwmeester H et al (2006) Trichome dynamics and artemisinin accumulation during development and senescence of Artemisia annua leaves. Planta Med 72:336–345
Lu X, Zhang L, Zhang F et al (2013) AaORA, a trichome-specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea. New Phytol 198:1191–1202
Lualon W, De-Eknamkul W, Tanaka H et al (2008) Artemisinin production by shoot regeneration of Artemisia annua L. using thidiazuron. Z Naturforsch C 63:96–100
Lv Z, Zhang L, Tang K (2017) New insights into artemisinin regulation. Plant Signal Behav 12:e1366398
Maes L, Van Nieuwerburgh FC, Zhang Y et al (2011) Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol 189:176–189
Mannan A, Shaheen N, Arshad W et al (2008) Hairy roots induction and artemisinin analysis in Artemisia dubia and Artemisia indica. Afr J Biotechnol 7:3288–3292
Mannan A, Ahmed I, Arshad W et al (2010a) Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malar J 9:310
Mannan A, Liu C, Arsenault PR et al (2010b) DMSO triggers the generation of ROS leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep 29:143–152
Matsushita Y, Kang W, Charlwood BV (1996) Cloning and analysis of a cDNA encoding farnesyl diphosphate synthase from Artemisia annua. Gene 172:207–209
Mercke P, Bengtsson M, Bouwmeester HJ et al (2000) Molecular cloning, expression, and characterization of amorpha-4, 11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381:173–180
Mostafa E, Towler MJ, Gazzinelli RT et al Use of whole plant Artemisia annua L. as an antimalarial therapy. In: UMass Center for Clinical and Translational Science Research Retreat, Hoagland Pincus Conference Center, 2012. p 46
Nahlen BL, Korenromp EL, Miller JM et al (2005) Malaria risk: estimating clinical episodes of malaria. Nature 437:E3-E3
Newman JD, Marshall J, Chang M et al (2006) High-level production of amorpha-4, 11-diene in a two-phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol Bioeng 95:684–691
Nguyen KT, Arsenault PR, Weathers PJ (2011) Trichomes + roots + ROS = artemisinin: regulating artemisinin biosynthesis in Artemisia annua L. In Vitro Cell Dev Biol Plant 47:329–338
Nguyen KT, Towler MJ, Weathers PJ (2013) The effect of roots and media constituents on trichomes and artemisinin production in Artemisia annua L. Plant Cell Rep 32:207–218
Noorden RV (2010) Demand for malaria drug soars. Nature 466:672–673
O’Neill PM, Barton VE, Ward SA (2010) The molecular mechanism of action of artemisinin-the debate continues. Molecules 15:1705–1721
Obolskiy D, Pischel I, Feistel B et al (2011) Artemisia dracunculus L. (Tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem 59:11367–11384
Oksman-Caldentey K-M, Sévon N (2002) Agrobacterium rhizogenes-mediated transformation: root cultures as a source of alkaloids. Planta Med 68:859–868
Olsson ME, Olofsson LM, Lindahl A-L et al (2009) Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry 70:1123–1128
Paddon CJ, Westfall PJ, Pitera D et al (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496:528–532
Palazón J, Cusidó RM, Gonzalo J et al (1998a) Relation between the amount of rolC gene product and indole alkaloid accumulation in Catharanthus roseus transformed root cultures. J Plant Physiol 153:712–718
Palazón J, Cusidó RM, Roig C et al (1998b) Expression of the rolC gene and nicotine production in transgenic roots and their regenerated plants. Plant Cell Rep 17:384–390
Paniego NB, Giulietti AM (1996) Artemisinin production by Artemisia annua L.-transformed organ cultures. Enzym Microb Technol 18:526–530
Peng MF, Chen M, Chen R et al (2011) The last gene involved in the MEP pathway of Artemisia annua: cloning and characterization and functional identification. J Med Plants Res 5:223–230
Picaud S, Olsson ME, Brodelius PE (2007) Improved conditions for production of recombinant plant sesquiterpene synthases in Escherichia coli. Protein Expr Purif 51:71–79
Rasool R, Ganai BA, Akbar S et al (2013) Variation in bioactive principles of Artemisia amygdalina Decne. in wild and tissue culture regenerants. Pak J Pharm Sci 26:623–628
Ro D-K, Paradise EM, Ouellet M et al (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Sa G, Mi M, He-Chun Y et al (2001) Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci 160:691–698
Schmülling T, Fladung M, Grossmann K et al (1993) Hormonal content and sensitivity of transgenic tobacco and potato plants expressing single rol genes of Agrobacterium rhizogenes T-DNA. Plant J 3:371–382
Shaneeja V, Keshavachandran R, James P et al (2014) Genetic transformation in Artemesia annua L. for hairy root induction and enhancement of secondary metabolites. J Trop Agric 52:149–153
Sharma P, Padh H, Shrivastava N (2013) Hairy root cultures: a suitable biological system for studying secondary metabolic pathways in plants. Eng Life Sci 13:62–75
Shkryl YN, Veremeichik GN, Bulgakov VP et al (2008) Individual and combined effects of the rolA, B, and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol Bioeng 100:118–125
Singh ND, Kumar S, Daniell H (2016) Expression of β-glucosidase increases trichome density and artemisinin content in transgenic Artemisia annua plants. Plant Biotechnol J 14:1034–1045
Sivakumar G, Liu C, Towler MJ et al (2010) Biomass production of hairy roots of Artemisia annua and Arachis hypogaea in a scaled-up mist bioreactor. Biotechnol Bioeng 107:802–813
Slezakova S, Ruda-Kucerova J (2017) Anticancer activity of artemisinin and its derivatives. Anticancer Res 37:5995–6003
Smith TC, Weathers PJ, Cheetham RD (1997) Effects of gibberellic acid on hairy root cultures of Artemisia annua: growth and artemisinin production. In Vitro Cell Dev Biol Plant 33:75–79
Souret FF, Weathers PJ, Wobbe KK (2002) The mevalonate-independent pathway is expressed in transformed roots of Artemisia annua and regulated by light and culture age. In Vitro Cell Dev Biol Plant 38:581–588
Souret FF, Kim Y, Wyslouzil BE et al (2003) Scale-up of Artemisia annua L. hairy root cultures produces complex patterns of terpenoid gene expression. Biotechnol Bioeng 83:653–667
Tan H, Xiao L, Gao S et al (2015) TRICHOME AND ARTEMISININ REGULATOR 1 is required for trichome development and artemisinin biosynthesis in Artemisia annua L. Mol Plant 8:1396–1411
Tang K, Shen Q, Yan T et al (2014) Transgenic approach to increase artemisinin content in Artemisia annua L. Plant Cell Rep 33:605–615
Tayyab Ansari M, Saeed Saify Z, Sultana N et al (2013) Malaria and artemisinin derivatives: an updated review. Mini-Rev Med Chem 13:1879–1902
Tellez MR, Canel C, Rimando AM et al (1999) Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochemistry 52:1035–1040
Teoh KH, Polichuk DR, Reed DW et al (2006) Artemisia annua L.(Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580:1411–1416
Trovato M, Maras B, Linhares F et al (2001) The plant oncogene rolD encodes a functional ornithine cyclodeaminase. Proc Natl Acad Sci USA 98:13449–13453
Valentine D, Witkowski B, Ménard D (2016) Plasmodium falciparum resistance to artemisinin derivatives and piperaquine: a major challenge for malaria elimination in Cambodia. Am J Trop Med Hyg 95:1228–1238
Wallaart TE, Bouwmeester HJ, Hille J et al (2001) Amorpha-4, 11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Wang Y, Weathers P (2007) Sugars proportionately affect artemisinin production. Plant Cell Rep 26:1073–1081
Wang H, Liu Y, Chong K et al (2007) Earlier flowering induced by over-expression of CO gene does not accompany increase of artemisinin biosynthesis in Artemisia annua. Plant Biol 9:442–446
Wang Y, Jing F, Yu S et al (2011) Co-overexpression of the HMGR and FPS genes enhances artemisinin content in Artemisia annua L. J Med Plants Res 5:3396–3403
Wang S, Towler MJ, Weathers PJ (2016) Root regulation of artemisinin production in Artemisia annua: trichome and metabolite evidence. Planta 244:999–1010
Weathers PJ, Towler MJ (2012) The flavonoids casticin and artemetin are poorly extracted and are unstable in an Artemisia annua tea infusion. Planta Med 78:1024–1026
Weathers P, Cheetham R, Follansbee E et al (1994) Artemisinin production by transformed roots of Artemisia annua. Biotechnol Lett 16:1281–1286
Weathers P, Hemmavanh D, Walcerz D et al (1997) Interactive effects of nitrate and phosphate salts, sucrose, and inoculum culture age on growth and sesquiterpene production in Artemisia annua hairy root cultures. In Vitro Cell Dev Biol Plant 33:306–312
Weathers P, DeJesus-Gonzalez L, Kim Y et al (2004) Alteration of biomass and artemisinin production in Artemisia annua hairy roots by media sterilization method and sugars. Plant Cell Rep 23:414–418
Weathers P, Bunk G, McCoy M (2005) The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell Dev Biol Plant 41:47–53
Weathers PJ, Elkholy S, Wobbe KK (2006) Artemisinin: the biosynthetic pathway and its regulation in Artemisia annua, a terpenoid-rich species. In Vitro Cell Dev Biol Plant 42:309–317
Weathers PJ, Arsenault PR, Covello PS et al (2011) Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 10:173–183
Weathers P, Towler M, Wang Y et al (2012) Artemisinin: controlling its production in Artemisia annua. In: Bach TJ, Rohmer M (eds) Isoprenoid synthesis in plants and microorganisms. Springer, New York, pp 107–117
Weathers PJ, Towler M, Hassanali A et al (2014) Dried-leaf Artemisia annua: a practical malaria therapeutic for developing countries? World J Pharmacol 3:39–55
WHO (2017) World Malaria Report 2017
Yan L, He-Chun Y, Hong W et al (2003) Molecular cloning, Escherichia coli expression and genomic organization of squalene synthase gene from Artemisia annua. Acta Bot Sin 45:608–613
Yin H, Kjaer A, Fretté XC et al (2012) Chitosan oligosaccharide and salicylic acid up-regulate gene expression differently in relation to the biosynthesis of artemisinin in Artemisia annua L. Process Biochem 47:1559–1562
Zhang Y, Teoh KH, Reed DW et al (2008) The molecular cloning of artemisinic aldehyde ∆11 (13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283:21501–21508
Zhu C, Cook SP (2012) A concise synthesis of (+)-artemisinin. J Am Chem Soc 134:13577–13579
Acknowledgements
Higher Education Commission of Pakistan (HEC) is acknowledged for financial support.
Author information
Authors and Affiliations
Contributions
WKK and BM conceived and designed the study. WKK, BHK, and ED wrote the manuscript. BM supervised all the experiments. WKK and BM reviewed the manuscript. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kayani, W.K., Kiani, B.H., Dilshad, E. et al. Biotechnological approaches for artemisinin production in Artemisia. World J Microbiol Biotechnol 34, 54 (2018). https://doi.org/10.1007/s11274-018-2432-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2432-9