Abstract
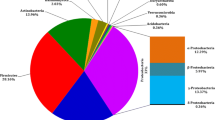
Banana is one of the most important fruits cultivated in Malaysia, and it provides many health benefits. However, bacterial wilt disease, which attacks bananas, inflicts major losses on the banana industry in Malaysia. To understand the complex interactions of the microbiota of bacterial wilt-diseased banana plants, we first determined the bacterial communities residing in the pseudostems of infected (symptomatic) and diseased-free (non-symptomatic) banana plants. We characterized the associated microorganisms using the targeted 16S rRNA metagenomics sequencing on the Illumina MiSeq platform. Taxonomic classifications revealed 17 and nine known bacterial phyla in the tissues of non-symptomatic and symptomatic plants, respectively. Cyanobacteria and Proteobacteria (accounted for more than 99% of the 16S rRNA gene fragments) were the two most abundant phyla in both plants. The five major genera found in both plant samples were Ralstonia, Sphingomonas, Methylobacterium, Flavobacterium, and Pseudomonas. Ralstonia was more abundant in symptomatic plant (59% out of the entire genera) as compared to those in the non-symptomatic plant (only 36%). Our data revealed that 102 bacterial genera were only assigned to the non-symptomatic plant. Overall, this study indicated that more diverse and abundant microbiota were associated with the non-symptomatic bacterial wilt-diseased banana plant as compared to the symptomatic plant. The higher diversity of endophytic microbiota in the non-symptomatic banana plant could be an indication of pathogen suppression which delayed or prevented the disease expression. This comparative study of the microbiota in the two plant conditions might provide caveats for potential biological control strategies.
Graphical Abstract





Similar content being viewed by others
References
Adams DG, Duggan PS (2012) Signalling in cyanobacteria–plant symbioses. In: Perotto S, Baluska F (eds) Signaling and communication in plant symbiosis. Springer, Berlin, pp 93–121
Andreote FD, Azevedo JL, Araújo WL (2009) Assessing the diversity of bacterial communities associated with plants. Brazilian J Microbiol 40:417–432
Andrews S (2010) FASTQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed 29 Sept 2015
BAPNET Bulletin (2012) Bacterial wilt builds up in Malaysia. Official Newsletter of the Banana Asia Pacific Network. Bioversity International, vol 18. https://www.researchgate.net/profile/Mohamad_roff_Mohd_Noor/publication/313800600_Bacterial_wilt_builds_up_in_Malaysia/. Accessed 13 Jan 2016
Bartram AK, Lynch MD, Stearns JC, Moreno-Hagelsieb G, Neufeld JD (2011) Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Appl Environ Microbiol 77:3846–3852
Berg G, Grube M, Schloter M, Smalla K (2015) Unraveling the plant microbiome: looking back and future perspectives. Front Microbiol. doi:10.3389/fmicb.2014.00148
Bulgari D, Casati P, Crepaldi P, Daffonchio D, Quaglino F, Brusetti L, Bianco PA (2011) Restructuring of endophytic bacterial communities in grapevine yellows-diseased and recovered Vitis vinifera L. plants. Appl Environ Microbiol 77:5018–5022
Coenye T (2014) The family Burkholderiaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The Prokaryotes. Springer, Berlin, pp 759–776
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Devine SP, Pelletreau KN, Rumpho ME (2012) 16S rDNA-based metagenomic analysis of bacterial diversity associated with two populations of the kleptoplastic sea slug Elysia chlorotica and its algal prey Vaucheria litorea. Biol Bull 223:138–154
Fan H, Gulley ML (2001) DNA extraction from fresh or frozen tissues. In: Killeen AA (ed) Molecular pathology protocols. Springer, Berlin, pp 5–10
Fegan M, Prior P (2006) Diverse members of the Ralstonia solanacearum species complex cause bacterial wilts of banana. Australas Plant Pathol 35:93–101. doi:10.1071/ap05105
Harish S, Kavino M, Kumar N, Saravanakumar D, Soorianathasundaram K, Samiyappan R (2008) Biohardening with plant growth promoting rhizosphere and endophytic bacteria induces systemic resistance against Banana bunchy top virus. Appl Soil Ecol 39:187–200. doi:10.1016/j.apsoil.2007.12.006
Harish S, Kavino M, Kumar N, Balasubramanian P, Samiyappan R (2009) Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Biol Control 51:16–25
Huson DH, Mitra S, Ruscheweyh H-J, Weber N, Schuster SC (2011) Integrative analysis of environmental sequences using MEGAN4. Genome Res 21:1552–1560
Innerebner G, Knief C, Vorholt JA (2011) Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl Environ Microbiol 77:3202–3210
Koberl M, Dita M, Martinuz A, Staver C, Berg G (2015) Agroforestry leads to shifts within the Gammaproteobacterial microbiome of banana plants cultivated in Central America. Front Microbiol. doi:10.3389/fmicb.2015.00091
Köberl M, Dita M, Martinuz A, Staver C, Berg G (2017) Members of Gammaproteobacteria as indicator species of healthy banana plants on Fusarium wilt-infested fields in Central America. Sci Rep. doi:10.1038/srep45318
Lamichhane JR, Venturi V (2015) Synergisms between microbial pathogens in plant disease complexes: a growing trend. Front Plant Sci 6:385
Leach JE (2015) The phytobiome initiative. Plant and Animal Genome XXIII Conference. Plant and Animal Genome
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37(5):634–663
Mokhtarud-din H, William R (2011) Status of banana cultivation and disease incidences in Malaysia. In: Abstract of the Workshop on Integrated Approaches in Banana Disease Management, 22 March 2011, p 5
Muthoni J, Shimelis H, Melis R (2012) Management of Bacterial Wilt [Rhalstonia solanacearum Yabuuchi et al. 1995] of Potatoes: opportunity for Host Resistance in Kenya. J Agric Sci 4:64
Paret ML, Kubota R, Jenkins DM, Alvarez AM (2010) Survival of Ralstonia solanacearum race 4 in drainage water and soil and detection with immunodiagnostic and DNA-based assays. Hort Technol 20:539–548
Pini F, Frascella A, Santopolo L, Bazzicalupo M, Biondi EG, Scotti C, Mengoni A (2012) Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol 12:78
Reiter B, Pfeifer U, Schwab H, Sessitsch A (2002) Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Appl Environ Microbiol 68:2261–2268
Rossmann B, Müller H, Smalla K, Mpiira S, Tumuhairwe JB, Staver C, Berg G (2012) Banana-associated microbial communities in Uganda are highly diverse but dominated by Enterobacteriaceae. Appl Environ Microbiol 78:4933–4941
Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U (2014) Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: Proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov. R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol 64:3087–3103
Sanchez Perez A, Mejia L, Fegan M, Allen C (2008) Diversity and distribution of Ralstonia solanacearum strains in Guatemala and rare occurrence of tomato fruit infection. Plant Pathol 57:320–331
Santoyo G, Orozco-Mosqueda MdC, Govindappa M (2012) Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Technol 22:855–872
Saravanakumar D, Harish S, Loganathan M, Vivekananthan R, Rajendran L, Raguchander T, Samiyappan R (2007) Rhizobacterial bioformulation for the effective management of Macrophomina root rot in mungbean. Arch Phytopathol Plant Protect 40:323–337
Scholthof K-BG (2007) The disease triangle: pathogens, the environment and society. Nat Rev Microbiol 5:152–156
Sika KC et al (2015) A simple and efficient genomic DNA extraction protocol for large scale genetic analyses of plant biological systems. Plant Gene 1:43–45
Sivasakthi S, Usharani G, Saranraj P (2014) Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: a review. Afr J Agric Res 9:1265–1277
Souza H, Muller L, Brandão R, Lovato M (2012) Isolation of high quality and polysaccharide-free DNA from leaves of Dimorphandra mollis (Leguminosae), A tree from the Brazilian Cerrado. Genet Mol Res 11:756–764
Suhaimi NSM, Laboh R, Ajam N, Thong KL (2016) Antagonistic effects of biofilm-forming bacterial strains co-inoculated with blood disease pathogenic strain, Ralstonia syzygii subspecies celebesensis in banana plants. Eur J Plant Pathol 148(1):13–26
Teng S-K et al (2016) The occurrence of blood disease of Banana in Selangor, Malaysia. Int J Agric Biol. doi:10.17957/IJAB/15.0067
Timin JA, Irene L, Saifful Bahri AM (2014) Occurrence of Banana Blood Disease in Malaysia. In: Abstract of the 8th International Conference on Plant Protection in the Tropics, 8–10 April 2014
Zhai Y, Wang W, Tan H, Cao L (2016) A new approach to analyzing endophytic actinobacterial population in the roots of banana plants (Musa sp., AAA). J Biochem Mol Biol Res 2(3):180–184
Zulperi D, Sijam K (2014) First report of Ralstonia solanacearum race 2 biovar 1 causing Moko disease of banana in Malaysia. Plant Dis 98(2):275–275
Acknowledgements
We thank University of Malaya for facilities and support. This project was supported by Postgraduate Research Fund, University of Malaya (No. PV123-2012A awarded to NSMS) and High Impact Research Grants, Ministry of Higher Education (MOHE), Malaysia (No. UM.C/625/1HIR/MOHE/-02 awarded to TKL and H-50001-A000027 awarded to CKG). The first author was financially supported by MOHE, Malaysia and SLAI Scheme, University of Malaya. We would like to acknowledge officers and field-workers from Crop Protection and Plant Quarantine Unit, Department of Agriculture, also the banana smallholder in Negeri Sembilan, Malaysia for giving permission to conduct the sampling of plant materials and assistance in sampling processes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11274_2017_2336_MOESM1_ESM.pdf
Online Resource 1—Cladogram of bacteria associated with Non-Symptomatic (NS) and Symptomatic (SP) plants tissue samples at the Phylum level. (PDF 85 KB)
11274_2017_2336_MOESM2_ESM.pdf
Online Resource 2—Cladogram of bacteria associated with Non-Symptomatic (NS) and Symptomatic (SP) plants tissue samples at the Order level. (PDF 146 KB)
11274_2017_2336_MOESM3_ESM.pdf
Online Resource 3—Cladogram of bacteria associated with Non-Symptomatic (NS) and Symptomatic (SP) tissue samples at the Genus level. (PDF 238 KB)
Rights and permissions
About this article
Cite this article
Suhaimi, N.S.M., Goh, SY., Ajam, N. et al. Diversity of microbiota associated with symptomatic and non-symptomatic bacterial wilt-diseased banana plants determined using 16S rRNA metagenome sequencing. World J Microbiol Biotechnol 33, 168 (2017). https://doi.org/10.1007/s11274-017-2336-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2336-0