Abstract
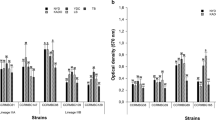
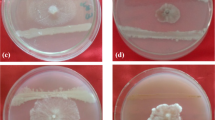
A blood disease pathogenic strain, Ralstonia syzygii subspecies celebesensis was used to study the possible association of biofilm-forming bacteria with the development and severity of blood disease in banana plants. Therefore, the objective of this study was to determine the effects of mono-culture and co-culture inoculation of isolated biofilm-forming bacteria with the blood disease pathogen in banana pseudostems in glasshouse conditions. Putative biofilm-forming bacteria were isolated from an infected banana plant and were further identified using 16SrRNA sequencing. Four isolates, identified as Enterobacter hormaechei, Enterobacter cloacae, Kosakonia radicincitans and Klebsiella pneumoniae, were inoculated as a mono- and co-culture with R. syzygii subsp. celebesensis into 2 months old banana plants. The observation after the 8 weeks of post inoculation showed that plants which were co-inoculated with the pathogen and K. radicincitans, a biofilm-forming bacterium, were the most susceptible towards the infection. In contrast, plants under two treatments (which were co-inoculated with the pathogen and E. cloacae and the pathogen with E. hormaechei) were less susceptible towards the infection. This study revealed the antagonistic effects of two biofilm-forming strains which reduced the severity of infection caused by the pathogenic agent. Scanning electron micrographs of the cross section of plant rhizomes indicated the dissimilarity of adhesion and host colonization conditions of the pathogen in each infected plant from different treatments.





Similar content being viewed by others
References
Álvarez, B., Biosca, E. G., & López, M. M. (2010). On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. Current Research, Technology and Education Topics in Applied Microbiology And Microbial Biotechnology, 1, 267–279.
Bairu, M. W., Aremu, A. O., & Van Staden, J. (2011). Somaclonal variation in plants: causes and detection methods. Plant Growth Regulation, 63, 147–173.
Barka, E. A., Gognies, S., Nowak, J., Audran, J.-C., & Belarbi, A. (2002). Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biological Control, 24, 135–142.
Beer, S. (1988). Biological control of Pythium seed rot and preemergence damping-off of cotton with Enterobacter cloacae and Erwinia herbicola applied as seed treatments. Plant Disease 72.
Bogino, P. C., Oliva, M. D. L. M., Sorroche, F. G., & Giordano, W. (2013). The role of bacterial biofilms and surface components in plant-bacterial associations. International Journal of Molecular Sciences, 14, 15838–15859.
Brady, C., Cleenwerck, I., Venter, S., Coutinho, T., & De Vos, P. (2013). Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Systematic and Applied Microbiology 36, 309.
Charkowski, A. O., Lind, J., & Rubio-Salazar, I. (2014). Genomics of plant-associated bacteria: the soft rot Enterobacteriaceae. Genomics of Plant-Associated Bacteria. Springer, p. 37–58.
Chelvam, K. K., Chai, L. C., & Thong, K. L. (2014). Variations in motility and biofilm formation of Salmonella enterica serovar Typhi. Gut Pathogens, 6, 2.
Collins, D. P., & Jacobsen, B. J. (2003). Optimizing a Bacillus subtilis isolate for biological control of sugar beet cercospora leaf spot. Biological Control, 26, 153–161.
Compant, S., Duffy, B., Nowak, J., Clément, C., & Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology, 71, 4951–4959.
Emaldi, U., Trujillo, I., & de García, E. (2004). Comparison of characteristics of bananas (Musa sp.) from the somaclone CIEN BTA-03 and its parental clone Williams. Fruits, 59, 257–263.
Fegan, M., & Prior, P. (2006). Diverse members of the Ralstonia solanacearum species complex cause bacterial wilts of banana. Australasian Plant Pathology, 35, 93–101.
Fouts, D. E., Tyler, H. L., DeBoy, R. T., Daugherty, S., Ren, Q., et al. (2008). Complete genome sequence of the N2-fixing broad host range Endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS Genetics, 4(7), e1000141.
Genin, S., & Boucher, C. (2002). Ralstonia solanacearum: secrets of a major pathogen unveiled by analysis of its genome. Molecular Plant Pathology, 3, 111–118.
Jacob, B. M., & Kim, E. (2010). Inhibiting biofilm formation of Enterobacter sp. prevented premature withering in cut flowers. Korean Journal of Chemical Engineering, 27, 1252–1257.
Kang, Y., Liu, H., Genin, S., Schell, M. A., & Denny, T. P. (2002). Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Molecular Microbiology, 46, 427–437.
Kogeethavani, R., Sulastri, N.J., Mazanah, M., Rozeita, L. & Mohamad Roff, M. N. (2013). First report of blood disease bacterium on banana in Malaysia. Malaysian Agricultural Research and Development Institute.
Koutsoudis, M. D., Tsaltas, D., Minogue, T. D., & von Bodman, S. B. (2006). Quorum-sensing regulation governs bacterial adhesion, biofilm development, and host colonization in Pantoea stewartii subspecies stewartii. Proceedings of the National Academy of Sciences, 103, 5983–5988.
Mak, C., Mohamed, A. A., Liew, K. W., & Ho, Y. W. (2004). Early screening technique for fusarium wilt resistance in banana micropropagated plants. Banana Improvement: Cellular, Molecular Biology, and Induced Mutations 219–227.
Melcher, U., Verma, R., & Schneider, W. L. (2014). Metagenomic search strategies for interactions among plants and multiple microbes. Frontiers in Plant Science 5.
Meneses, C. H., Rouws, L. F., Simões-Araújo, J. L., Vidal, M. S., & Baldani, J. I. (2011). Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Molecular Plant-Microbe Interactions, 24, 1448–1458.
Misas‐Villamil, J. C., Kolodziejek, I., & van der Hoorn, R. A. (2011). Pseudomonas syringae colonizes distant tissues in Nicotiana benthamiana through xylem vessels. The Plant Journal, 67, 774–782.
Muthoni, J., Shimelis, H., & Melis, R. (2012). Management of bacterial wilt [Ralstonia solanacearum Yabuuchi et al., 1995] of potatoes: opportunity for host resistance in Kenya. Journal of Agricultural Science, 4, 64.
Muyzer, G., Teske, A., Wirsen, C. O., & Jannasch, H. W. (1995). Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Archives of Microbiology, 164, 165–172.
Ramesh, R., Joshi, A., & Ghanekar, M. (2009). Pseudomonads: major antagonistic endophytic bacteria to suppress bacterial wilt pathogen, Ralstonia solanacearum in the eggplant (Solanum melongena L.). World Journal of Microbiology and Biotechnology, 25, 47–55.
Remenant, B., de Cambiaire, J. C., Cellier, G., Jacobs, J. M., Mangenot, S., Barbe, V., et al. (2011). Ralstonia syzygii, the blood disease bacterium and some Asian R. solanacearum strains form a single genomic species despite divergent lifestyles. Plos One 6.
Safni, I., Cleenwerck, I., De Vos, P., Fegan, M., Sly, L., & Kappler, U. (2014). Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. International Journal of Systematic and Evolutionary Microbiology, 64, 3087–3103.
Scarparo, C., Piccoli, P., Ricordi, P., & Scagnelli, M. (2002). Comparative evaluation of two commercial chromogenic media for detection and presumptive identification of urinary tract pathogens. European Journal of Clinical Microbiology and Infectious Diseases, 21, 283–289.
Stepanović, S., Vuković, D., Dakić, I., Savić, B., & Švabić-Vlahović, M. (2000). A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of Microbiological Methods, 40, 175–179.
Vasse, J., Danoun, S., Trigalet, A., Allen, C., Prior, P., & Hayward, A. (2005). Microscopic studies of root infection in resistant tomato cultivar Hawaii7996. Bacterial wilt Disease and the Ralstonia Solanacearum Species Complex, 285–291.
Villa, J., Tsuchiya, K., Horita, M., Natural, M., Opina, N., & Hyakumachi, M. (2003). DNA analysis of Ralstonia solanacearum and related bacteria based on 282-bp PCR-amplified fragment. Plant Disease, 87, 1337–1343.
Acknowledgments
We thank University of Malaya for facilities and support. This research was supported by University of Malaya IPPP grant (PV123/2012A). The first author is supported by Ministry of Higher Education, Malaysia and SLAI Scheme, University of Malaya. We would like to thank Nur Sulastri binti Jaffar for the use of R. syzygii subsp. celebesensis strain. We also acknowledge officer and staff from the Plant and Soil Science Research Center, MARDI Serdang, Selangor for providing generous help and guidance through the glasshouse experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suhaimi, N.S.M., Laboh, R., Ajam, N. et al. Antagonistic effects of biofilm-forming bacterial strains co-inoculated with blood disease pathogenic strain, Ralstonia syzygii subspecies celebesensis in banana plants. Eur J Plant Pathol 148, 13–26 (2017). https://doi.org/10.1007/s10658-016-1064-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1064-x