Abstract
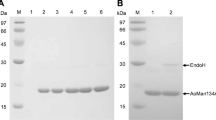
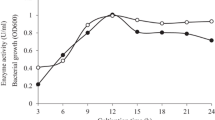
An alkaline-thermostable mannanase from Streptomyces sp. CS428 was produced, purified, and biochemically characterized. The extracellular mannanase (Mn428) was purified to homogeneity with 12.4 fold, specific activity of 2406.7 U/mg, and final recovery of 37.6 %. The purified β-mannanase was found to be a monomeric protein with a molecular mass of approximately 35 kDa as analyzed by SDS-PAGE and zymography. The first N-terminal amino acid sequences of mannanase enzyme were HIRNGNHQLPTG. The optimal temperature and pH for enzyme were 60 °C and 12.5, respectively. The mannanase activities were significantly affected by the presence of metal ions, modulators, and detergents. Km and Vmax values of Mn428 were 1.01 ± 3.4 mg/mL and 5029 ± 85 µmol/min mg, respectively when different concentrations (0.6–10 mg/mL) of locust bean gum galactomannan were used as substrate. The substrate specificity of enzyme showed its highest specificity towards galactomannan which was further hydrolyzed to produce mannose, mannobiose, mannotriose, and a series of mannooligosaccharides. Mannooligosaccharides can be further converted to ethanol production, thus the purified β-mannanase isolated from Streptomyces sp. CS428 was found to be attractive for biotechnological applications.




Similar content being viewed by others
References
Al-Ghazzewi FH, Tester RF (2012) Efficacy of cellulase and mannanase hydrolysates of konjac glucomannan to promote the growth of lactic acid bacteria. J Sci Food Agric 92:2394–2396
Arcand N, Kluepfel D, Paradis FW, Morosoli R, Shareck F (1993) Beta-mannanase of Streptomyces lividans 66: cloning and DNA sequence of the manA gene and characterization of the enzyme. Biochem J 290(Pt 3):857–863
Blibech M, Ghorbel RE, Fakhfakh I, Ntarima P, Piens K, Bacha AB, Ellouz CS (2010) Purification and characterization of a low molecular weight of β-mannanase from Penicillium occitanis Pol6. App Biochem Biotechnol 160:1227–1240
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chandra M, Lee YS, Park IH, Zhou Y, Kim KK, Choi Y (2011) Isolation, purification and characterization of a thermostable β-mannanase from Paenibacillus sp. DZ3. J Korean Soc Appl Biol Chem 54:325–331
Chauhan PS, Puri N, Sharma P, Gupta N (2012) Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol 93:1817–1830
Chauhan P, Sharma P, Puri N, Gupta N (2014) Purification and characterization of an alkali-thermostable β-mannanase from Bacillus nealsonii PN-11 and its application in mannooligosaccharides preparation having prebiotic potential. Eur Food Res Technol 238:927–936
Chernin LS, Winson MK, Thompson JM, Haran S, Bycroft BW, Chet I, Williams P, Stewart GS (1998) Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J Bacteriol 180:4435–4441
Daniel RM, Dines M, Petach HH (1996) The denaturation and degradation of stable enzymes at high temperatures. Biochem J 317(Pt 1):1–11
Duff SJB, Murray WD (1996) Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour Technol 55:1–33
Eneyskaya EV, Sundqvist G, Golubev AM, Ibatullin FM, Ivanen DR, Shabalin KA, Brumer H, Kulminskaya AA (2009) Transglycosylating and hydrolytic activities of the β-mannosidase from Trichoderma reesei. Biochimie 91:632–638
Hatada Y, Takeda N, Hirasawa K, Ohta Y, Usami R, Yoshida Y, Grant WD, Ito S, Horikoshi K (2005) Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in Bacillus subtilis and characterization of the recombinant enzyme. Extremophiles 9:497–500
Jiang Z, Wei Y, Li D, Li L, Chai P, Kusakabe I (2006) High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr Polym 66:88–96
Kansoh AL, Nagieb ZA (2004) Xylanase and mannanase enzymes from Streptomyces galbus NR and their use in biobleaching of softwood kraft pulp. Anton Van Leeuw 85:103–114
Kim DY, Ham SJ, Lee HJ, Cho HY, Kim JH, Kim YJ, Shin DH, Rhee YH, Son KH, Park HY (2011) Cloning and characterization of a modular GH5 β-1,4-mannanase with high specific activity from the fibrolytic bacterium Cellulosimicrobium sp. strain HY-13. Bioresour Technol 102:9185–9192
Kumagai Y, Usuki H, Yamamoto Y, Yamasato A, Arima J, Mukaihara T, Hatanaka T (2011) Characterization of calcium ion sensitive region for β-mannanase from Streptomyces thermolilacinus. Biochim Biophys Acta Protein Proteom 1814:1127–1133
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Ma Y, Xue Y, Dou Y, Xu Z, Tao W, Zhou P (2004) Characterization and gene cloning of a novel beta-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 8:447–454
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Montiel M, Hernandez M, Rodriguez J, Arias M (2002) Evaluation of an endo-β-mannanase produced by Streptomyces ipomoea CECT 3341 for the biobleaching of pine kraft pulps. Appl Microbiol Biotechnol 58:67–72
Pradeep GC, Choi YH, Choi YS, Seong CN, Cho SS, Lee HJ, Yoo JC (2013) A novel thermostable cellulase free xylanase stable in broad range of pH from Streptomyces sp. CS428. Proc Biochem 48:1188–1196
Pradeep GC, Choi YH, Choi YS, Suh SE, Seong JH, Cho SS, Bae MS, Yoo JC (2014) An extremely alkaline novel chitinase from Streptomyces sp. CS495. Proc Biochem 49:223–229
Puchart V, Vrsanska M, Svoboda P, Pohl J, Ogel ZB, Biely P (2004) Purification and characterization of two forms of endo-β-1,4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). Biochim Biophys Acta Gen Subj 1674:239–250
Sachslehner A, Haltrich D (1999) Purification and some properties of a thermostable acidic endo-β-1,4-d-mannanase from Sclerotium (Athelia) rolfsii. FEMS Microbiol Lett 177:47–55
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Singh G, Bhalla A, Hoondal GS (2010) Solid state fermentation and characterization of partially purified thermostable mannanase from Bacillus sp. MG-33. BioResour 5:1689–1701
Takahashi R, Kusakabe I, Kobayashi H, Murakami K, Maekawa A, Suzuki T (1984) Purification and some properties of mannanase from Streptomyces sp. Agric Biol Chem 48:2189–2195
Tamaru Y, Araki T, Amagoi H, Mori H, Morishita T (1995) Purification and characterization of an extracellular beta-1,4-mannanase from a marine bacterium, Vibrio sp. strain MA-138. Appl Environ Microbiol 61:4454–4458
Titapoka S, Keawsompong S, Haltrich D, Nitisinprasert S (2008) Selection and characterization of mannanase-producing bacteria useful for the formation of prebiotic manno-oligosaccharides from copra meal. World J Microbiol Biotechnol 24:1425–1433
Vazquez MJ, Alonso JL, DomıiNguez H, Parajo JC (2000) Xylooligosaccharides: manufacture and applications. Trend Food Sci Technol 11:387–393
Wang J, Shao Z, Hong Y, Li C, Fu X, Liu Z (2010) A novel β-mannanase from Pantoea agglomerans A021: gene cloning, expression, purification and characterization. World J Microbiol Biotechnol 26:1777–1784
Ward OP, Moo YM (1988) Thermostable enzymes. Biotechnol Adv 6:39–69
Zakaria MM, Yamamoto S, Yagi T (1998) Purification and characterization of an endo-1,4-β-mannanase from Bacillus subtilis KU-1. FEMS Microbiol Lett 158:25–31
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2015R1A2A1A15056120, NRF-2015R1D1A1A 010 59 483) and Bio-industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (115073-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Pradeep G. C. and Seung Sik Cho have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pradeep G. C., Cho, S.S., Choi, Y.H. et al. An extremely alkaline mannanase from Streptomyces sp. CS428 hydrolyzes galactomannan producing series of mannooligosaccharides. World J Microbiol Biotechnol 32, 84 (2016). https://doi.org/10.1007/s11274-016-2040-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2040-5