Abstract
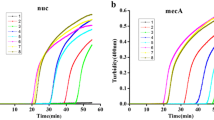
Extracting DNA from Staphylococcus aureus cells is important for detecting MRSA by PCR. However, S. aureus cells are known to be difficult to disrupt due to their compact cell walls. Here, we systematically studied the efficiency of a highly active lysin ClyH for extracting DNA of S. aureus in comparison with commonly used enzymes, such as lysostaphin and achromopeptidase (ACP), and its compatibility in quantitative PCR (qPCR) detection of MRSA. qPCR analysis of S. aureus specific gene femB showed that ClyH was much faster than lysostaphin, ACP and lysozyme for releasing DNA. Five minutes disruption with ClyH at room temperature was enough to release all the DNA from S. aureus. Analysis of the spiked nasal swabs by a dual qPCR assay of the β-lactam resistance mecA gene and the staphylococcal cassette chromosome (SCCmec)–open reading frame X (orfX) junction (SCCmec–orfX) after ClyH lysis showed 100 % sensitivity and specificity to the commercial BD GeneOhm™ MRSA test with ACP lysis, but the lysis time was reduced from 20 min by ACP to 5 min by ClyH. Our research shows that ClyH could be a better option than the currently used enzymes for DNA extraction from S. aureus, which can provide simpler and faster PCR detection of MRSA.



Similar content being viewed by others
References
Ablain W, Soulier SH, Causeur D, Gautier M, Baron F (2009) A simple and rapid method for the disruption of Staphylococcus aureus, optimized for quantitative reverse transcriptase applications: application for the examination of Camembert cheese dairy. Sci Technol 89:69–81. doi:10.1051/dst/2008034
Aldous WK, Pounder JI, Cloud JL, Woods GL (2005) Comparison of six methods of extracting Mycobacterium tuberculosis DNA from processed sputum for testing by quantitative real-time PCR. J Clin Microbiol 43:2471–2473. doi:10.1128/jcm.43.5.2471-2473.2005
Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, Gotz F (2007) Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol 189:280–283. doi:10.1128/jb.01221-06
Blot S, Vandewoude K, Colardyn F (1998) Staphylococcus aureus infections. N Engl J Med 339:2025–2026
Botaro BG, Cortinhas CS, Marco LV, Moreno JFG, Silva LFP, Benites NR, Santos MV (2013) Detection and enumeration of Staphylococcus aureus from bovine milk samples by real-time polymerase chain reaction. J Dairy Sci 96:6955–6964. doi:10.3168/jds.2013-6559
CLSI (2009) Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline, third edition. CLSI document M39-A3. Clinical Laboratory and Standards Institute, Wayne
Coffey B, Mills S, Coffey A, McAuliffe O, Ross RP (2010) Phage and their lysins as biocontrol agents for food safety applications. Annu Rev Food Sci Technol 1:449–468. doi:10.1146/annurev.food.102308.124046
Courchesne NM, Parisien A, Lan CQ (2009) Production and application of bacteriophage and bacteriophage-encoded lysins. Recent Pat Biotechnol 3:37–45
Fenton M, Ross P, McAuliffe O, O’Mahony J, Coffey A (2010) Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. doi:10.4161/bbug.1.1.9818
Fischetti VA (2008) Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400. doi:10.1016/j.mib.2008.09.012
Fischetti VA (2010) Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:357–362. doi:10.1016/j.ijmm.2010.04.002
Grundling A, Schneewind O (2006) Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J Bacteriol 188:2463–2472. doi:10.1128/jb.188.7.2463-2472.2006
Hohnadel M, Felden L, Fijuljanin D, Jouette S, Chollet R (2014) A new ultrasonic high-throughput instrument for rapid DNA release from microorganisms. J Microbiol Methods 99:71–80. doi:10.1016/j.mimet.2014.02.004
Hwang KY et al (2012) Solid phase DNA extraction with a flexible bead-packed microfluidic device to detect methicillin-resistant Staphylococcus aureus in nasal swabs. Anal Chem 84:7912–7918. doi:10.1021/ac3016533
Kim JU, Cha CH, An HK, Lee HJ, Kim MN (2013) Multiplex real-time PCR assay for detection of methicillin-resistant Staphylococcus aureus (MRSA) strains suitable in regions of high MRSA endemicity. J Clin Microbiol 51:1008–1013. doi:10.1128/jcm.02495-12
Kocagoz T, Yilmaz E, Ozkara S, Kocagoz S, Hayran M, Sachedeva M, Chambers HF (1993) Detection of Mycobacterium tuberculosis in sputum samples by polymerase chain-reaction using a simplified procedure. J Clin Microbiol 31:1435–1438
Liesack W, Weyland H, Stackebrandt E (1991) Potential risks of gene amplification by PCR as determined by 16S rDNA analysis of a mixed-culture of strict barophilic bacteria. Microb Ecol 21:191–198. doi:10.1007/bf02539153
Loeffler JM, Nelson D, Fischetti VA (2001) Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172. doi:10.1126/science.1066869
Loessner MJ (2005) Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol 8:480–487. doi:10.1016/j.mib.2005.06.002
Ma K et al (2014) Rapid and simultaneous detection of Salmonella, Shigella, and Staphylococcus aureus in fresh pork using a multiplex real-time PCR assay based on immunomagnetic separation. Food Control 42:87–93. doi:10.1016/j.foodcont.2014.01.042
McCann CD, Jordan JA (2014) Evaluation of MolYsis (TM) Complete5 DNA extraction method for detecting Staphylococcus aureus DNA from whole blood in a sepsis model using PCR/pyrosequencing. J Microbiol Methods 99:1–7. doi:10.1016/j.mimet.2014.01.013
Meng X et al (2011) Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl Environ Microbiol 77:8272–8279. doi:10.1128/aem.05151-11
Middelberg AP (1995) Process-scale disruption of microorganisms. Biotechnol Adv 13:491–551
Murray PR, Baron EJ (2003) Manual of clinical microbiology, 8th edn. American Society for Microbiology Press, Washington
Nelson D, Loomis L, Fischetti VA (2001) Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci USA 98:4107–4112. doi:10.1073/pnas.061038398
Niwa T, Kawamura Y, Katagiri Y, Ezaki T (2005) Lytic enzyme, labiase for a broad range of Gram-positive bacteria and its application to analyze functional DNA/RNA. J Microbiol Methods 61:251–260. doi:10.1016/j.mimet.2004.12.006
Oblath EA, Henley WH, Alarie JP, Ramsey JM (2013) A microfluidic chip integrating DNA extraction and real-time PCR for the detection of bacteria in saliva. Lab Chip 13:1325–1332. doi:10.1039/c3lc40961a
Rantakokko-Jalava K, Jalava J (2002) Optimal DNA isolation method for detection of bacteria in clinical specimens by broad-range PCR. J Clin Microbiol 40:4211–4217
Rashel M et al (2007) Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis 196:1237–1247. doi:10.1086/521305
Schuch R, Nelson D, Fischetti VA (2002) A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884–889. doi:10.1038/nature01026
Shan G, Jin W, Lam EK, Xing X (2008) Purification of total DNA extracted from activated sludge. J Environ Sci (China) 20:80–87
Shittu A, Nubel U, Udo E, Lin J, Gaogakwe S (2009) Characterization of meticillin-resistant Staphylococcus aureus isolates from hospitals in KwaZulu-Natal province. Repub S Afr J Med Microbiol 58:1219–1226. doi:10.1099/jmm.0.011452-0
Tiemersma EW et al (2004) Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis 10:1627–1634
von Wintzingerode F, Gobel UB, Stackebrandt E (1997) Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21:213–229
Wang HY, Kim S, Kim J, Park SD, Uh Y, Lee H (2014) Multiplex real-time PCR assay for rapid detection of methicillin-resistant staphylococci directly from positive blood cultures. J Clin Microbiol 52:1911–1920
Yang H, Zhang Y, Yu J, Huang Y, Zhang XE, Wei H (2014) Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 58:536–542. doi:10.1128/aac.01793-13
Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ (2012) Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 7:e33865. doi:10.1371/journal.pone.0033865
Acknowledgments
This work was supported by the basic research program of the Ministry of Science and Technology of China (2012CB721102 to JP Yu), the Chinese Academy of Sciences (Grant No.: KJZD-EW-L02), and the Key Laboratory of Special Pathogens and Biosafety, Wuhan Institute of Virology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Yang, H., Wang, J. et al. Comparison between a chimeric lysin ClyH and other enzymes for extracting DNA to detect methicillin resistant Staphylococcus aureus by quantitative PCR. World J Microbiol Biotechnol 32, 1 (2016). https://doi.org/10.1007/s11274-015-1971-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-015-1971-6