Abstract
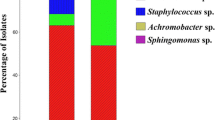
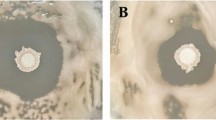
The fungus Rhizoctonia solani is one of the causal agents of numerous diseases that affect crop growth and yield. The aim of this present investigation was to identify a biocontrol agent that acts against R. solani and to determine the agent’s protective effect through phytohormones and antioxidant regulation in experimentally infected Chinese cabbage plants. Four rhizospheric soil bacterial isolates GR53, GR169, GR786, and GR320 were tested for their antagonistic activity against R. solani. Among these isolates, GR53 significantly suppressed fungal growth. GR53 was identified as Bacillus amyloliquefaciens subsp. plantarum by phylogenetic analysis of the 16S rDNA sequence. The biocontrol activity of B. amyloliquefaciens subsp. plantarum GR53 was tested in Chinese cabbage plants under controlled conditions. Results showed that R. solani inhibited plant growth (length, width, fresh and dry weight of leaves) by reducing chlorophyll and total phenolic content, as well as by increasing the levels of salicylic acid, jasmonic acid, abscisic acid, and DPPH scavenging activity. By regulating the levels of these compounds, the co-inoculation of B. amyloliquefaciens subsp. plantarum GR53 heightened induced systemic resistance in infected Chinese cabbage, effectively mitigating R. solani-induced damaging effects and improving plant growth. The results obtained from this study suggest that B. amyloliquefaciens subsp. plantarum GR53 is an effective biocontrol agent to prevent the damage caused by R. solani in Chinese cabbage plants.





Similar content being viewed by others
References
Adachi M, Sako Y, Ishida Y (1996) Analysis of Alexandrium (Dinophyceae) species using sequences of the 5.8 S ribosomal DNA and internal transcribed spacer regions. J Phycol 32:424–432
Amerine MA, Ough CS (1980) Methods for analysis of musts and wine. Wiley, New York, pp 205–206
Asaka O, Shoda M (1996) Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol 62:4081–4085
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Benhamou N, Kloepper JW, Quadt-Hallman A, Tuzon S (1996) Induction of defense-related ultrastructural modification in pea root tissues inoculated with endophytic bacteria. Plant Physiol 112:919–929
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Buonaurio R, Scarponi L, Ferrara L, Sidoti P, Bertona A (2002) Induction of systemic acquired resistance in pepper by acibenzolar-S-methyl against bacterial spot disease. Eur J Plant Pathol 108:41–49
Chatterjee A, Ghosh SK (2008) Alterations in biochemical components in mesta plants infected with yellow vein mosaic disease. Braz J Plant Physiol 20:267–275
Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262:1883–1886
Choudhary DK (2011) Plant growth promotion (PGP) activities and molecular characterization of rhizobacterial strains isolated from soybean (Glycine max L. Merril) plants against charcoal rot pathogen, Macrophomina phaseolina. Biotechnol Lett 33:2287–2295
Chowdhury SP, Dietel K, Randler M, Schmid M, Junge H, Borriss R, Hartmann A, Grosch R (2013) Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 8:e68818
Chung SH, Kim SD (2005) Biological control of phytopathogenic fungi by Bacillus amyloliquefaciens 7079; suppression rates are better than popular chemical fungicides. J Microbiol Biotechnol 15:1011–1021
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:49–51
Correa OS, Montecchia MS, Berti MF, Ferrari MCF, Pucheu NL, Kerber NL, Garcia AF (2009) Bacillus amyloliquefaciens BNM122, a potential microbial biocontrol agent applied on soybean seeds, causes a minor impact on rhizosphere and soil microbial communities. Appl Soil Ecol 41:185–194
Dixon RA, Harrison MG (1992) Activation, structure and organization of genes involved in microbial defense in plants. Adv Genet 28:165–234
Enyedi AJ, Yalpani N, Silverman P, Raskin I (1992) Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA 89:2480–2484
Gara LD, de-Pinto MC, Tommasi F (2003) The antioxidant systems vis-a-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol Biochem 41:863–870
Gasoni L, Gurfinkel BSD (2009) Biocontrol of Rhizoctonia solani by the endophytic fungus Cladorrhinum foecundissimum in cotton plants. Australas J Plant Pathol 38:389–391
Gonzalez-Garcia V, Portal-Onco MA, Rubio-Susan V (2006) Review: biology and systematics of the form genus Rhizoctonia. Span J Agric Res 4:55–79
Hadar Y, Chet I, Henis Y (1979) Biological control of Rhizoctonia solani damping-off with wheat bran culture of Trichoderma harzianum. Phytopathology 69:64–68
He H, Silo-Suh LA, Handelsman J, Clardy J (1994) Zwittermicin A, an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett 35:2499–2502
Huang X, Zhang N, Yong X, Yang X, Shen Q (2012) Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res 167:135–143
Ji SH, Paul NC, Deng JX, Kim YS, Yun BS, Yu SH (2013) Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 41:234–242
Johnsen K, Jacobsen C, Torsvik V, Sorensen J (2001) Pesticide effects on bacterial diversity in agricultural soils—a review. Biol Fertil Soils 33:443–453
Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ (2014) Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol 54:427–433
Lee J, Nam J, Park HC, Na G, Miura K, Jin JB, Yoo CY, Baek D, Kim DH, Jeong JC, Kim D, Lee SY, Salt DE, Mengiste T, Gong Q, Ma S, Bohnert HJ, Kwak SS, Bressan RA, Hasegawa PM, Yun DJ (2006) Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J 49:79–90
Lee MK, Chun JH, Byeon DH, Chung SO, Park SU, Park S, Arasu MV, Al-Dhabif NA, Limg YP, Kim SJ (2014) Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT Food Sci Technol 58:93–101
Loake G, Grant M (2007) Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol 10:466–472
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signaling network. Curr Opin Plant Biol 8:532–540
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant–pathogen interactions. Curr Opin Plant Biol 8:409–414
McCloud ES, Baldwin IT (1997) Herbivory and caterpillar regurgitants amplify the wound induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta 203:430–435
Mercado-Blanco J, van-der-Drift KM, Olsson PE, Thomas-Oates JE, van-Loon LC, Bakker PA (2001) Analysis of the pmsCEAB gene cluster involved in biosynthesis of salicylic acid and the siderophore pseudomonine in the biocontrol strain Pseudomonas fluorescens WCS374. J Bacteriol 183:1909–1920
Michael RY, Nannette YY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Mohr PG, Cahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30:461–469
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Naegely SK (1997) Greenhouse vegetables: business is booming. Greenh Grow 15:14–18
Nagarajkumar M, Bhaskaran R, Velazhahan R (2004) Involvement of secondary metabolites and extracellular lytic enzymes produced by Pseudomonas fluorescens in inhibition of Rhizoctonia solani, the rice sheath blight pathogen. Microbiol Res 159:73–81
Niewiadomska A (2004) Effect of carbendazim, imazetapir and thiram on nitrogenase activity, the number of microorganisms in soil and yield of red clover (Trifolium pratense L.). Pol J Environ Stud 13:403–410
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Osman MS, Sivakumar D, Korsten L (2011) Effect of biocontrol agent Bacillus amyloliquefaciens and 1-methylcyclopropene on the control of postharvest diseases and maintenance of fruit quality. Crop Prot 30:173–178
Pavlo A, Leonid O, Iryna Z, Natalia K, Maria PA (2011) Endophytic bacteria enhancing growth and disease resistance of potato (Solanum tuberosum L.). Biol Control 56:43–49
Qi QG, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ (1998) (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very long chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol 117:979–987
Radhakrishnan R, Shim KB, Lee BW, Hwang CD, Pae SB, Park CH, Kim SU, Lee CK, Baek IY (2013) IAA-producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.). J Microbiol Biotechnol 23:856–863
Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12:1633–1646
Reithner B, Schuhmacher R, Stoppacher N, Pucher M, Brunner K, Zeilinger S (2007) Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk1 differentially affects mycoparasitism and plant protection. Fungal Genet Biol 44:1123–1133
Rezzonico E, Flury N, Meins FJ, Beffa R (1998) Transcriptional downregulation by abscisic acid of pathogenesis-related beta-1,3-glucanase genes in tobacco cell cultures. Plant Physiol 117:585–592
Seskar M, Shulaev V, Raskin I (1998) Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol 116:387–392
Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Shiau FL, Chung WC, Huang JW, Huang HC (1999) Organic amendment of commercial culture media for improving control of Rhizoctonia damping-off of cabbage. Can J Plant Pathol 21:368–374
Sneh B, Ichielevich-Auster M, Plaut Z (1989) Mechanisms of seedling protection by a hypovirulent isolate of Rhizoctonia solani. Can J Bot 67:2135–2141
Solanki MK, Kumar S, Pandey AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocont Sci Technol 22:203–217
Stabb EV, Jacobson LM, Handelsman J (1994) Zwittermicin A producing strains of Bacillus cereus from diverse soils. Appl Environ Microbiol 60:4404–4412
Thaler SJ, Bostock RM (2004) Interactions between abscisic-acid mediated responses and plant resistance to pathogens and insects. Ecology 85:48–58
Thimmaiah SR (1999) Standard method of biochemical analysis. Kalyani Publishers, New Delhi, pp 230–231
Vicente MR, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Wassternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Acknowledgments
This work was financially supported by National Research Foundation of Korea (NRF), Ministry of Science, ICT and Future Planning through Basic Science Research Program (2014R1A1A1004918).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, SM., Radhakrishnan, R. & Lee, IJ. Bacillus amyloliquefaciens subsp. plantarum GR53, a potent biocontrol agent resists Rhizoctonia disease on Chinese cabbage through hormonal and antioxidants regulation. World J Microbiol Biotechnol 31, 1517–1527 (2015). https://doi.org/10.1007/s11274-015-1896-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1896-0